Matching
|
|
|
Match each item with the correct statement below. a. | proton | d. | electron | b. | nucleus | e. | neutron | c. | atom |
|
|
|
1.
|
a subatomic particle with no charge that is found in the nucleus
|
|
|
2.
|
the smallest particle of an element that is comprised of smaller
particles.
|
|
|
3.
|
the central part of an atom, containing protons and neutrons
|
|
|
4.
|
a negatively charged subatomic particle found outside the nucleus
|
|
|
5.
|
a positively charged subatomic particle that is found in the nucleus
|
|
|
Match each item with the correct statement below. a. | mass number | c. | atomic number | b. | isotopes | d. | atomic mass |
|
|
|
6.
|
atoms with the same number of protons, but different numbers of neutrons in the
nucleus of an atom
|
|
|
7.
|
the number of protons in the nucleus of an element
|
|
|
8.
|
atoms of the same element with different numbers of neutrons
|
|
|
9.
|
the total number of protons and neutrons in the nucleus of an atom
|
|
|
Match each item with the correct statement below. a. | group | c. | Mendeleev | b. | period | d. | Moseley |
|
|
|
10.
|
horizontal row in the periodic table
|
|
|
11.
|
vertical column in the periodic table
|
|
|
12.
|
created the first periodic table and predicted undiscovered elements
|
|
|
13.
|
reordered the periodic table in order of increasing atomic number
|
Multiple Choice
Identify the choice that best completes the
statement or answers the question.
|
|
|
14.
|
An atom has 12 protons and 13 neutrons. Which is the correct chemical symbol for
this atom?
|
|
|
|
|
|
15.
|
What is the mass number of the atom shown above?
|
|
|
16.
|
Which is the correct symbol for this atom shown above?
|
|
|
17.
|
The above figure is of a/an:
a. | anion | b. | isotope | c. | cation | d. | subion |
|
|
|
18.
|
Which element has 14 protons?
|
|
|
19.
|
Which is the number of neutrons in 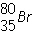 ?
|
|
|
20.
|
Elements within a veritcal GROUP on the periodic table have similar properties
because:
a. | they have the same number of valence electrons. | b. | they have the same
the same number of energy levels. | c. | they have the same number of
protons. | d. | they have the same number of neutrons. |
|
|
|
21.
|
Most NONMETALS are
a. | ductile. | b. | good conductors of heat and
electricity. | c. | gases at room temperature | d. | malleable. |
|
|
|
22.
|
Which group of elements shares characteristics with both metals and nonmetals
and are used to make semiconductors?
a. | salts | b. | metalloids | c. | halogens | d. | alloys |
|
|
|
23.
|
Elements that are in the same PERIOD
a. | have the same number of valence electrons. | b. | have different
numbers of energy levels. | c. | have the same number of energy
levels. | d. | have similar properties. |
|
Numeric Response
Place the following in the numerical order of
discovery (1 being the earliest- 5 being the most recent):
|
|
|
24.
|
_____ Bohr- determined that electrons reside in energy levels that surround the
nucleus
|
|
|
25.
|
_____ Rutherford: Discovered the positive nucleus and that atoms were mostly
empty space.
|
|
|
26.
|
_____ Quantum mechanical model- Electrons reside
within orbits in energy levels whose location is based on probability
|
|
|
27.
|
_____ Thomson- discovered the electron. Placed
electrons randomly in a positive surrounding (blueberry muffin model)
|
|
|
28.
|
_____ Dalton- Atoms were solid speres with no smaller particles (like
marbles)
|
|
|
|
Use the following for Questions 29-35
When Answering, MAKE SURE THAT YOU MATCH THE LETTER CASE EXACTLY
|
|
|
29.
|
Which of the Symbols SHOWN is that of an Alkali Metal?
|
|
|
30.
|
Which of the Symbols SHOWN is that of a Noble Gas?
|
|
|
31.
|
Which of the Symbols SHOWN is that of a Halogen?
|
|
|
32.
|
Which of the Symbols SHOWN is that of an Alkaline Earth Metal?
|
|
|
33.
|
Which of the Symbols SHOWN is that of a Metalloidl?
|
|
|
34.
|
Which of the following will GAIN electrons to be like the nearest noble gas, Na
or F?
|
|
|
35.
|
Which of the following will form a CATION, Na or F?
|