Matching
|
|
|
Match each item with the correct statement
below. a. | mass | c. | Celsius temperature scale | b. | Kelvin temperature scale | d. | weight |
|
|
|
1.
|
kilogram is the SI base unit for this quantity
|
|
|
2.
|
the non-SI scale for temperature
|
|
|
3.
|
the SI scale for temperature
|
|
|
4.
|
the force of gravity on an object
|
Multiple Choice
Identify the choice that best completes the
statement or answers the question.
|
|
|
5.
|
Multiply 5.0 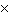 10 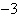 and
8.0 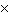 10 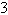 and
report the answer in CORRECT scientific notation.
|
|
|
6.
|
The density of mercury is 5,427 kg/(m3).
If the density of water is 1.0 g/mL, will mercury float or sink in water?
a. | Mercury will sink because the density of mercury is
5.427 g/mL, which is greater than the 1.0 g/mL density of water. | b. | Mercury will float because the density of mercury is 0.005427 g/mL, which is
less than the 1.0 g/mL density of water. | c. | Mercury will sink
because the density of mercury is 5,427 g/mL, which is greater than the 1.0 g/mL density of
water. | d. | Mercury will float because the density of mercury is
0.05427 g/mL, which is less than the 1.0 g/mL density of
water. |
|
|
|
7.
|
What is the volume of 80.0 g of ether if the
density of ether is 0.70 g/mL?
|
|
|
8.
|
Which of the following conversion factors would you
use to change 18 kilometers to meters?
a. | 1 km/1000 m | c. | 1000
m/1 km | b. | 100 m/1 km | d. | 1 km/100
m |
|
|
|
9.
|
The standard base unit for mass is
the
a. | gram. | b. | kilogram. | c. | meter. | d. | cubic
centimeter. |
|
|
|
10.
|
On the Celsius scale, at what temperature does
water boil?
|
|
|
11.
|
Which of the following mass units is the
largest?
|
|
|
12.
|
A conversion factor that shows the relationship
between grams and kilograms is
|
|
|
13.
|
Which is the correct measurement for gray bar shown below?  a. | 1.365 | b. | 1.36 | c. | 1.036 | d. | 1.30 |
|
|
|
14.
|
A change in the force of gravity on an object will
affect its
a. | weight. | b. | kinetic energy. | c. | mass. | d. | density. |
|
|
|
15.
|
In the lab, a student measured the length of an item to be 1.53 m, which digit
is the estimated digit?
a. | none are estimated | c. | 1 | b. | 3 | d. | 5 |
|
|
|
16.
|
What is 1 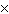 10 3 / 4 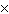
10 -3 in CORRECT scientific notation? [divide] a. | .25 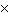 100 100 | b. | 2.5 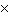 106
106 | c. | 2.5 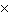 10-5 10-5 | d. | 2.5 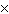 105
105 |
|
|
|
17.
|
An object has a mass of 26.94 grams and a volume of 2.568
cubic centimeters. What material is it likely to be made of?
| Substance | iron | gold | silver | copper | | Density (g/cm3) | 7.874 | 19.32 | 10.49 | 8.92 | | | | | |
a. | Silver | b. | Gold | c. | Iron | d. | Copper |
|
|
|
18.
|
The symbol mm represents a
a. | micrometer. | c. | manometer. | b. | megameter. | d. | millimeter. |
|
|
|
19.
|
The symbols for units of length in order from
largest to smallest are
a. | m, cm, mm, km. | c. | km,
mm, cm, m. | b. | km, m, cm,
mm. | d. | mm, m, cm, km. |
|
|
|
20.
|
The SI base unit used to measure mass is
the
a. | kilogram. | c. | meter. | b. | second. | d. | liter. |
|
|
|
21.
|
How is 0.00099 written in scientific notation?
a. | 99 ´ 10–5 | b. | 9.9 ´ 104 | c. | 0.99 ´ 10–3 | d. | 9.9 ´ 10–4 |
|
|
|
22.
|
Which pair of quantities determines the density of
a material?
a. | volume and weight | c. | volume
and mass | b. | volume and concentration | d. | mass and
weight |
|
|
|
23.
|
Which safety measure should you take for every
single laboratory experiment?
a. | Follow your teacher’s
instructions. | b. | Open all classroom
windows. | c. | Wear a lab apron. | d. | Use heat-resistant gloves. |
|
|
|
24.
|
Express the product of 4.0 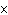 10
10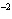 m and 8.1 m and 8.1 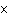 10 10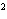 m using the correct number of significant digits. m using the correct number of significant digits.
|
|
|
25.
|
All of the following are examples of units
except
a. | kilometer. | b. | mass. | c. | ounce. | d. | gram. |
|
|
|
26.
|
A beaker contains 0.5 L of water. What is the volume of this water in
milliliters (1 L = 1000 mL)?
a. | 0.5 mL | b. | 5 mL | c. | 5000 mL | d. | 500
mL |
|
|
|
27.
|
Which of the following shows good precision but poor accuracy? 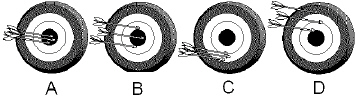
|
|
|
28.
|
The product of (2 ´ 104) x (4 ´ 10–6), expressed in scientific notation is ____.
a. | 6 ´ 10-24 | b. | 8 ´
1010 | c. | 8 ´ 10–2 | d. | 8 ´ 10–24 |
|
|
|
29.
|
The density of aluminum is 2.70 g/cm3.
What is the mass of a solid piece of aluminum with a volume of 1.50 cm3?
a. | 0.556 g | b. | 4.05
g | c. | 4.20 g | d. | 1.80
g |
|
|
|
30.
|
Which of these is not an SI base
unit?
a. | second | b. | kilogram | c. | liter | d. | Kelvin |
|
|
|
31.
|
The SI base units for length and time
are
a. | centimeter and hour. | c. | meter and second. | b. | centimeter and
second. | d. | meter and hour. |
|
|
|
32.
|
What is the density of an object having a mass of
8.0 g and a volume of 25 cm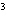 ? ?
a. | 0.32 g/cm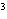 | b. | 2.0 g/cm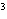 | c. | 200
g/cm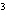 | d. | 3.1
g/cm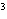 |
|
|
|
33.
|
100 is equal to
|
|
|
34.
|
The chief advantage of the metric system over other
systems of measurement is that it ____.
a. | is in multiples of 10 | b. | is in multiples of 15 | c. | is derived from
nature itself | d. | has more
units |
|
|
|
35.
|
The diameter of a carbon atom is 0.000 000 000 154 m. What is this number
expressed in scientific notation?
|
|
|
36.
|
The unit m3 measures
a. | length. | b. | volume. | c. | area. | d. | time. |
|
|
|
37.
|
The radius of Earth is 6 370 000 m. Express this
measurement in km in scientific notation with the correct number of significant digits.
|
|
|
38.
|
Which is the correct measurement for location marked by the
arrow?
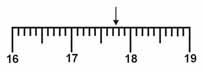
a. | 17.8 | b. | 17.7 | c. | 17.76 | d. | 17.076 |
|
|
|
39.
|
The expression of 8336 in scientific notation is ____.
|
|
|
40.
|
The density of an object is calculated
by
a. | dividing its volume by its mass. | c. | multiplying its mass times its volume. | b. | adding its mass to its volume. | d. | dividing its mass
by its volume. |
|