Matching
|
|
|
Match each item with the correct statement below. a. | halogens | d. | noble gases | b. | alkaline-earth metals | e. | alkali metals | c. | transition
metals |
|
|
|
1.
|
elements to the far right that are largely unreactive
|
|
|
2.
|
reactive elements of Group 17 that are poor conductors
|
|
|
3.
|
Ca, Mg and Sr
|
|
|
4.
|
elements that belong to Groups 3-12 and are somewhat reactive
|
|
|
5.
|
highly reactive elements that belong to Group 1
|
|
|
Match each item with the correct statement below. a. | ground state | c. | photon | b. | excited state |
|
|
|
6.
|
an electron that has just lost energy
|
|
|
7.
|
unit or quantum of light
|
|
|
8.
|
an electron when it gains energy
|
|
|
Match each item with the correct statement below. a. | proton | d. | electron | b. | nucleus | e. | neutron | c. | atom |
|
|
|
9.
|
a subatomic particle with no charge
|
|
|
10.
|
the smallest particle of an element that retains the properties of that
element
|
|
|
11.
|
a positively charged subatomic particle
|
|
|
12.
|
a negatively charged subatomic particle
|
|
|
13.
|
the central part of an atom, containing protons and neutrons
|
|
|
Match each item with the correct statement below. a. | mass number | d. | atomic mass | b. | atomic mass unit (amu) | e. | isotope | c. | atomic
number |
|
|
|
14.
|
1/12th the mass of a carbon atom having six protons and six
neutrons
|
|
|
15.
|
atoms with the same number of protons, but different numbers of neutrons in the
nucleus of an atom
|
|
|
16.
|
the total number of protons and neutrons in the nucleus of an atom
|
|
|
17.
|
the number of protons in the nucleus of an element
|
|
|
18.
|
the weighted average of the masses of the isotopes of an element
|
True / False indicate whether the statement is true or
false
|
|
|
19.
|
Noble Gases are located in group 17 on the periodic table.
|
|
|
20.
|
All atoms of the same element must have the same atomic number (# of
protons).
|
|
|
21.
|
The maximum number of electrons in the second energy level of an atom is
10.
|
|
|
22.
|
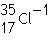 has a negative charge, therefore it is an
anion.
|
|
|
23.
|
Elements arranged in horizontal rows in the periodic table are called
periods.
|
Multiple Choice
|
|
|
24.
|
The particles of matter that have about the same mass as protons
____.
a. | atoms | b. | positrons | c. | isotopes | d. | neutrons |
|
|
|
25.
|
In which of the following is the number of neutrons correctly
represented?
a. | 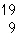 F has 28 neutrons. F has 28 neutrons. | c. | 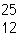 Mg has 13 neutrons. Mg has 13 neutrons. | b. | 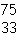 As has 108
neutrons. As has 108
neutrons. | d. | 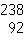 U
has 330 neutrons. U
has 330 neutrons. |
|
|
|
26.
|
What unit is used to measure weighted average atomic mass?
a. | amu | b. | kilogram | c. | angstrom | d. | nanogram |
|
|
|
27.
|
Two isotopes of carbon are 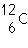 and 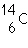 . If E is the symbol for an element, which two of the following symbols
represent isotopes of element
E?
1. 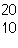 E
2. 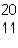 E 3. 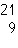 E 4. 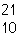 E a. | 1 and 2 | b. | 3 and 4 | c. | 1 and
4 | d. | 2
and 3 |
|
|
|
28.
|
Each energy level of an atom has a maximum number of ____ it can hold.
a. | sneutrons | b. | electron | c. | quarks | d. | protons |
|
|
|
29.
|
In a neutral atom the number of protons are equal to the number of ___.
a. | negatrons | b. | neutrons | c. | ions | d. | electrons |
|
|
|
30.
|
Rutherford's experiments led him to conclude that atoms contain massive
central regions that have
a. | a positive charge. | c. | no charge. | b. | a negative charge. | d. | both protons and
electrons. |
|
|
|
31.
|
An atom is electrically neutral because
a. | neutrons balance the protons and electrons. | b. | nuclear forces
stabilize the charges. | c. | the numbers of protons and electrons are
equal. | d. | the numbers of protons and neutrons are equal. |
|
|
|
32.
|
Isotopes are atoms of the same element that have different
a. | principal chemical properties. | c. | numbers of
protons. | b. | masses. | d. | numbers of electrons. |
|
|
|
33.
|
The tritium atom (Hydrogen-3) consists of
a. | one proton, two neutrons, and two electrons. | b. | one proton, one
neutron, and one electron. | c. | one proton, two neutrons, and one
electron. | d. | two protons, one neutron, and one electron. |
|
|
|
34.
|
All isotopes of hydrogen contain
a. | one neutron. | c. | one proton. | b. | two electrons. | d. | two nuclei. |
|
|
|
35.
|
Carbon-14 (atomic number 6), the radioactive nuclide used in dating fossils,
has
a. | 6 neutrons. | c. | 10 neutrons. | b. | 8 neutrons. | d. | 14 neutrons. |
|
|
|
36.
|
The number of atoms in 1 mol of carbon is
a. | 12 | b. | 6.02  1023. 1023. | c. | 6 | d. | 1 |
|
|
|
37.
|
The atomic number of neon is 10. The atomic number of calcium is 20. Compared
with a mole of neon, a mole of calcium contains
a. | twice as many atoms. | c. | an equal number of atoms. | b. | half as many
atoms. | d. | 20 times as many
atoms. |
|
|
|
38.
|
To determine the molar mass of an element, one must know the
element's
a. | Avogadro constant. | c. | number of isotopes. | b. | atomic number. | d. | average atomic
mass. |
|
|
|
39.
|
Molar mass
a. | is the mass in grams of one mole of a substance. | b. | is numerically equal
to the average atomic mass of the element. | c. | Both (a) and (b) | d. | Neither (a) nor
(b) |
|
|
|
40.
|
The mass of 2.0 mol of oxygen atoms (atomic mass 16.00 amu) is
a. | 16 g. | b. | 32 g. | c. | 48 g. | d. | 64
g. |
|
|
|
41.
|
A prospector finds 39.39 g of pure gold (atomic mass 196.9665 amu). She
has
a. | 1.2 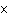 1023 atoms of Au. 1023 atoms of Au. | c. | 4.3 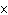 1023 atoms of Au.
1023 atoms of Au. | b. | 2.3 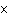 1023 atoms of
Au. 1023 atoms of
Au. | d. | 6.02 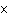 1023 atoms of Au.
1023 atoms of Au. |
|
|
|
42.
|
A sample of tin (atomic mass 118.71 amu) contains 3.01 ´ 1023
atoms. The mass of the sample is
a. | 3.01 g. | b. | 59.3 g. | c. | 72.6 g. | d. | 11
g. |
|
|
|
43.
|
If the atomic mass of carbon is 12 u, 1 mole of pure carbon will have a mass
of
a. | 6 g. | b. | 6 mol. | c. | 12 g. | d. | 12
mol. |
|
|
|
44.
|
Thomson is responsible for discovering that an atom contains
a. | electrons. | b. | molecules. | c. | anodes. | d. | a
nucleus. |
|
|
|
45.
|
What is the mass of 2.5 mol of Ca, which has a molar mass of 40 g/mol?
a. | 2.5 g Ca | b. | 20.0 g Ca | c. | 40.0 g Ca | d. | 100.0 g
Ca |
|
|
|
46.
|
A sodium atom, which has 11 electrons, has _____ electron(s) in its third energy
level.
|
|
|
47.
|
Avogadro’s number is defined as the number of particles in
a. | one mole of a substance. | c. | one gram of a
substance. | b. | one liter of a substance. | d. | one kilogram of a substance. |
|
|
|
48.
|
Molar mass is defined as
a. | the number of particles in one mole of a substance. | b. | the SI base unit
that describes the amount of a substance. | c. | the amount of a substance necessary to have a
positive charge. | d. | the mass in grams of one mole of a substance. |
|
|
|
49.
|
The person whose work led to a periodic table based on increasing atomic number
was
a. | Moseley. | b. | Mendeleev. | c. | Rutherford. | d. | Cannizzaro. |
|
|
|
50.
|
Which of the following elements is an alkali metal?
a. | calcium-Ca | b. | magnesium-Mg | c. | mercury-Hg | d. | sodium-Na |
|
|
|
51.
|
Most elements are
a. | metals. | c. | metalloids. | b. | nonmetals. | d. | semiconductors. |
|
|
|
|
|
|
52.
|
What is the mass number of the atom shown above?
|
|
|
53.
|
Which is the correct symbol for this atom shown above?
|
|
|
54.
|
All atoms of the same element
have the same ____.
a. | number of
neutrons | c. | mass
numbers | b. | number of protons | d. | mass |
|
|
|
55.
|
Which of the following sets of
symbols represents isotopes of the same element?
|