Matching
|
|
|
Match each item with the correct statement below. a. | metallic bond | c. | valence electron | b. | octet rule | d. | electron dot
structure |
|
|
|
1.
|
an electron in the highest occupied energy level of an atom
|
|
|
2.
|
Atoms react so as to acquire the stable electron structure of a noble
gas.
|
|
|
3.
|
a depiction of valence electrons around the symbol of an element
|
|
|
4.
|
the attraction of valence electrons for metal ions
|
Multiple Choice
Identify the choice that best completes the
statement or answers the question.
|
|
|
5.
|
The rule that sates that elements will gain, lose or share electrons in order to
have a full outer shell is called?
a. | None of these | b. | Foster’s rule | c. | Octet rule | d. | The Rule of
2 |
|
|
|
6.
|
How many electrons are needed in the outer energy levels of most atoms for the
atom to be chemically stable?
|
|
|
7.
|
What will happen to Oxygen, O when it bonds?
a. | lose 2 protons | b. | gain 2 electrons | c. | lose 2 electrons | d. | lose 6
electrons |
|
|
|
8.
|
What will happen to Magnesium , Mg when it bonds?
a. | gain 6 electrons | b. | gain 2 protons | c. | gain 2 electrons | d. | lose 2
electrons |
|
|
|
9.
|
Why do the noble gases NOT form compounds readily?
a. | They have no electrons. | b. | They have empty outer energy
levels. | c. | Their outer energy levels are completely filled with electrons. | d. | They have seven
electrons in the outer energy levels. |
|
|
|
10.
|
What kind of chemical bond is formed when a transfer of electrons occurs?
a. | ionic | b. | hydrate | c. | magnetic | d. | covalent |
|
|
|
11.
|
A chemical bond that occurs when atoms share electrons is a(n) ____ bond.
a. | polyatomic | b. | ionic | c. | covalent | d. | magnetic |
|
|
|
12.
|
Ionic compounds are held together by a force best compared
to________________?
a. | magnetic attraction | c. | both a and b | b. | electronic repulsion | d. | neither a or b |
|
|
|
13.
|
A double covalent bond contains _____ electrons?
|
|
|
14.
|
Bonds that form between two nonmetals are usually
a. | impossible. | b. | weak. | c. | ionic. | d. | covalent. |
|
|
|
15.
|
In an electron dot diagram, the dots are used to represent
a. | neutrons | c. | all electrons. | b. | valence electrons. | d. | protons |
|
|
|
16.
|
The formation of an ionic bond involves the
a. | sharing of electrons. | c. | transfer of electrons. | b. | transfer of
protons. | d. | transfer of
neutrons. |
|
|
|
17.
|
A chemical bond that occurs when atoms share electrons UNEQUALLY is a(n) ____
bond.
a. | polar covalent | c. | nonpolar covalent | b. | polar ionic | d. | polyatomic |
|
|
|
18.
|
A chemical bond that occurs when atoms share electrons EQUALLY is a(n) ____
bond.
a. | polyatomic | b. | polar | c. | nonpolar | d. | ionic |
|
|
|
19.
|
Which is true of the model of bonding shown in this figure? 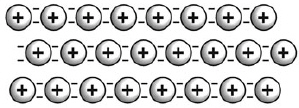 a. | Metallic atoms are present in a “sea” of negatively charged
atoms. | b. | Valence electrons are able to move easily among the metallic
cations. | c. | It results in the substance being very brittle and not easily
deformed. | d. | Heat and electricity are easily carried by the cations from one region to
another. |
|
|
|
20.
|
How many valence electrons are in an atom of phosphorus?
|
|
|
21.
|
How many valence electrons are in an atom of magnesium?
|
|
|
22.
|
How many valence electrons are in a silicon atom?
|
|
|
23.
|
How does oxygen obey the octet rule when reacting to form compounds?
a. | It gains electrons. | b. | It gives up electrons. | c. | It does not change
its number of electrons. | d. | Oxygen does not obey the octet
rule. |
|
|
|
24.
|
What characteristic of metals makes them good electrical conductors?
a. | They have mobile valence electrons. | b. | They have mobile protons. | c. | They have mobile
cations. | d. | Their crystal structures can be rearranged easily. |
|
|
|
25.
|
How many single covalent bonds can carbon form?
|
|
|
26.
|
How many single covalent bonds can halogens form?
|
|
|
27.
|
In the compound PCl3, how many total valence electrons are
present?
|
|
|
28.
|
Which type of bond has an electronegativity difference greater than 1.7?
a. | ionic | c. | nonpolar covalent | b. | metallic | d. | polar covalent |
|
|
|
29.
|
What molecular shape does the compound CCl4 have?
a. | trigonal planar | c. | octahedral | b. | tetrahedral | d. | bent |
|
|
|
30.
|
Fluorine belongs to group 7A / 17. How many covalent bonds are formed between
two fluorine atoms?
|
|
|
31.
|
If the electronegativity of H is 2.20 and of Cl is 3.55, which type of bond is
formed between H and Cl, when they form hydrogen chloride?
a. | Ionic | c. | Covalent | b. | Polar ionic | d. | Polar covalent |
|
|
|
32.
|
Which of the following contains a double bond? 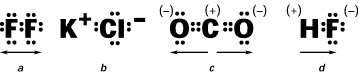
|
|
|
33.
|
Which of the following COVALENT compounds is the most polar? 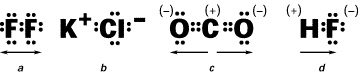
|
|
|
34.
|
Which of the following central atoms has a 2:0 ratio and has a linear
shape? 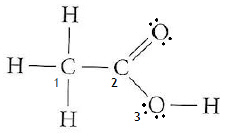
|