True/False
Indicate whether the statement is true or
false.
|
|
|
|
1.
|
All samples of the same substance have the same density.
|
|
|
|
2.
|
Objects with a density greater than water will sink in water.
|
|
|
|
3.
|
Density can be expressed in g/ml or g/cm3.
|
|
|
|
4.
|
Absolute Zero, 0K = 273°C
|
|
|
|
5.
|
Compounds are two or more substances which are not chemically
comined
|
|
|
|
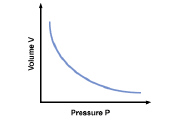
6.
|
The following graph shows a DIRECT relationship between Volume and
Pressure.
|
|
|
|
7.
|
Increasing the number of particles, heating, or decreasing volume will all cause
an increase in pressure.
|
|
|
|
8.
|
An isotope is an element with a positive or negative
charge.
|
|
|
|
9.
|
the resulting energy from electrons moving from lower to higher energy levels is
what causes the colored light that is produced in flame tests and neon lights.
|
|
|
|
10.
|
The modern periodic table (the one that we use now) is arranged in order of
increasing atomic number.
|
|
|
|
11.
|
Non-metals are located in the upper right hand side of the periodic
table.
|
|
|
|
12.
|
Ionic bonds are formed by the sharing of electrons.
|
|
|
|
13.
|
In a chemical reaction, the products are found on the left side of the
arrow.
|
|
|
|
14.
|
A precipitate forms when two aqueous solutions react to form a product that
cannot dissolve in water.
|
|
|
|
15.
|
Something that is insoluble will NOT dissolve in
water
|
|
|
|
16.
|
The solute is what dissolves the solvent in a solution.
|
|
|
|
17.
|
A pH value of 10 is considered to be neutral.
|
Matching
|
| |
|
|
Match the energy level with the maximum number of electrons that it can
hold.
|
|
|
|
18.
|
the third evergy level
|
|
|
|
19.
|
the first energy level
|
|
|
|
20.
|
the second energy level
|
Multiple Choice
Identify the choice that best completes the
statement or answers the question.
|
|
|
|
21.
|
Which of the following indicates a quantitative measurement?
a. | cold | c. | big | b. | 55.0 g | d. | yellow |
|
|
|
|
22.
|
Which of the following is a physical property?
a. | Melting point | c. | color | b. | density | d. | all of the
above |
|
|
|
|
23.
|
Which is the correct measurement for location marked by the arrow?
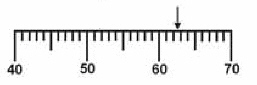 a. | 60.25 | c. | 62.7 | b. | 62 | d. | 62.659 |
|
|
|
|
24.
|
What is the measurement 0.000 024 cm in proper scientific
notation?
a. | 2.40 x 10-5cm. | c. | 240 x 10-4cm.
| b. | 2.40 x 10-4 cm. | d. | 2.40 x 10-6cm. |
|
|
|
|
25.
|
An object has a mass of 18.5 kg. What is the mass of the
object in
grams? (1000 g = 1 kg)
a. | 1.85 x 104 g | c. | 185 x 10-1
g | b. | 18.5 x 103 g | d. | 1.85 x 10-4 g |
|
|
|
|
26.
|
What is the volume of the following rock?
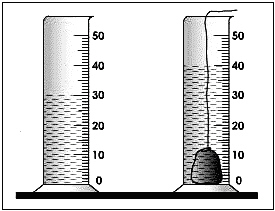 a. | 30 mL | c. | 70 mL | b. | 40 mL | d. | 10 mL |
|
|
|
|
27.
|
A group of students performed an experiment to calculate the mass of a
substance. The accepted value for the mass should be 2.85 grams. The group recorded the
following measurements after performing the experiment three times. Which statement correctly
describes the experimental data?Trial | Measurement | 1 | 2.01 | 2 | 3.05 | 3 | 2.75 | | |
a. | Precise but not accurate | c. | Accurate but not
precise | b. | Both accurate and precise | d. | Neither accurate nor precise |
|
|
|
|
28.
|
Silver, Copper and Oxygen are all:
a. | elements | c. | mixtures | b. | compounds | d. | metals |
|
|
|
|
29.
|
Which of the following is NOT a state of matter
a. | solid | c. | cold | b. | gas | d. | liquid |
|
|
|
|
30.
|
According to the Kinetic Molecular Theory, which of the following is not in
motion?
a. | solids | c. | gases | b. | liquids | d. | none of these are
correct. |
|
|
|
|
31.
|
Which of the following states of matter has the MOST amount of energy?
a. | solids | c. | gases | b. | liquids | d. | none of these have
energy. |
|
|
|
|
32.
|
The process by which particles gain enough energy at the surface of a
liquid to become a gas is known as?
a. | Evaporation | c. | Freezing | b. | Deposition | d. | Sublimation |
|
|
|
|
33.
|
Between which two points does thermal energy increase and
boiling occur for water?
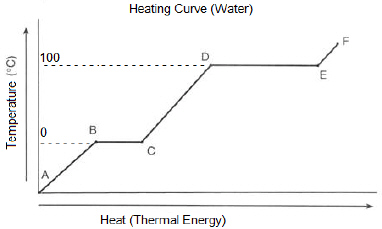 a. | A to B | c. | C to D | b. | B to C | d. | D to E |
|
|
|
|
34.
|
Accoriding to Boyle’s law, when volume decreases at a constant temperature
the following will happen:
a. | pressure stays the same | c. | pressure
increases | b. | pressure decreases | d. | none of the above |
|
|
|
|
35.
|
According to Bohr’s model of the atom, electrons must:
a. | not exist. | b. | be anywhere they want outside of the
nucleus. | c. | reside in the nucleus. | d. | move in “orbits” around the nucleus
like planets. |
|
|
|
|
36.
|
Which of the following are found inside the nucleus?
a. | protons | b. | neutrons | c. | electrons | d. | a and
b | e. | a
and c |
|
|
|
|
37.
|
The number of protons in an atom is always the same as it’s
a. | mass number | c. | isotope number | b. | atomic number | d. | nuetrons |
|
|
|
|
38.
|
Atoms are neutral when:
a. | protons and neutrons are equal | c. | protons and electrons are
equal | b. | neutrons and electrons are equal | d. | none of these are
correct. |
|
|
|
|
39.
|
24. Which of the following combinations describes an ion with a positive two
charge?
a. | 1 protons; 0 neutrons; 2 electrons | c. | 20 protons; 20 neutrons; 18
electrons | b. | 15 protons; 16 neutrons; 15 electrons | d. | 11 protons; 12 neutrons; 10
electrons |
|
|
|
|
40.
|
An atom’s mass number is the total of the:
a. | protons and neutrons | c. | protons and electrons | b. | neutrons and
electrons | d. | protons, neutrons
and electrons |
|
|
|
|
41.
|
Two neutral isotopes of the same element have a different number of:
a. | protons | b. | neutrons | c. | electrons | d. | a and
b | e. | a
and c |
|
|
|
|
42.
|
Which pair of symbols correctly represents two isotopes of the same
element?
a. |
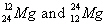 | c. |
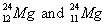 | b. |
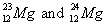 | d. | none of these represent
isotopes. |
|
|
|
|
43.
|
Which is the net charge on a particle that consists of 11 protons, 12
neutrons, and 10 electrons?
|
|
|
|
44.
|
Which is the mass number on a particle that consists of 11 protons, 12
neutrons, and 10 electrons?
|
|
|
|
45.
|
Which is the number of electrons in the Mg2+ ion?
|
|
|
|
46.
|
Lithium has an atomic mass of 6.9. which is the best explanation for
this?
a. | all lithium atoms have 6.9 mass units in their nuclei. | b. | The weighted average
of all lithium isotopes is 6.9. | c. | all lithium atoms have a mass of
6.9. |
|
|
|
|
47.
|
The amount of time that it takes for 1/2 of the mass of a radioactive isotope to
decay is known as a radioisotope’s:
a. | decay time | c. | half time | b. | radioactive time | d. | half life |
|
|
|
|
48.
|
The strongest type of radiation is known as:
a. | beta radiation | c. | alpha radiation | b. | gamma radiation | d. | delta radiation |
|
|
|
|
49.
|
Complete the following alha decay of Uranium-238
|
|
|
|
50.
|
Dimitri Mendeleev was known for his work on:
a. | I’ve never heard of him. | c. | the discovery of the
proton. | b. | his model of the atom | d. | the first periodic table. |
|
|
|
|
51.
|
Which of the following elements is most similar in properties to Nitrogen,
N?
|
|
|
|
52.
|
The energy level of the valence shell ist he same as the ________ that an
element can be found in.
a. | period number | c. | atomic number | b. | mass number | d. | group number |
|
|
|
|
53.
|
Element number 37 is a(n):
a. | halogen | c. | alkali metal | b. | transition metal | d. | alkaline earth
metal |
|
|
|
|
54.
|
Which of the following is a transition metal?
|
|
|
|
55.
|
Which of the following elements are BOTH noble gases?
a. | N and Ne | c. | Ne and Xe | b. | Cl and F | d. | H and He |
|
|
|
|
56.
|
From the top of the column 1 to the bottom of column 1 on the periodic
table:
a. | atomic radius decreases | c. | reactivity
decreases | b. | atomic mass decreases | d. | atomic radius increases |
|
|
|
|
57.
|
Which element will have the same number of electrons as
Ar when it forms a bond?
|
|
|
|
58.
|
Which of the following is the correct number of valence electrons for Ca?
|
|
|
|
59.
|
Which of the following has a full set of valence electrons?
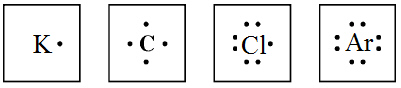
|
|
|
|
60.
|
How does K achieve a stable octet?
a. | it gains 1 electron | c. | it loses 1 electron | b. | it gains 7 electrons | d. | it does not do
anything |
|
|
|
|
61.
|
w does an ionically bonded compound such as, potassium chloride
(KCl) formed?
a. | A potassium atom loses an electron to chlorine to form an ionic
bond. | b. | a chlorine atom loses seven electrons to form a cation and potassium atom gains seven
electrons to form an ionic bond. | c. | potassium and chlorine do not form a
bond. |
|
|
|
|
62.
|
Which of the following is true for ionic compounds?
a. | high melting and boiling points | c. | usually solid at room
temperature | b. | conduct electricity in water | d. | all of the
above |
|
|
|
|
63.
|
In an ionic compound of potassium oxide, the potassium ion has a charge
of 1+ and the oxide ion has a charge of 2-. Which of the following is the
formula for potassium oxide?
|
|
|
|
64.
|
A structural formula with a triple covalent bond will have how many dashes
between two atoms?
|
|
|
|
65.
|
What is the name of LiF?
a. | lithium fluoride | c. | lithium monofluoride | b. | monolithium monofluoride | d. | Monolithium
fluoride |
|
|
|
|
66.
|
The type of formula that uses dashes to represent bonds is known as what type of
formula? Example:
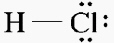 a. | math formula | c. | dash formula | b. | structure formula | d. | dot formula |
|
|
|
|
67.
|
How many oxygens are shown in the formula?:
Mg3(PO4)2
|
|
|
|
68.
|
What is the molar mass of NaOH?
|
|
|
|
69.
|
Complete the following statement of equality: 1 mol N = _?_ grams N = 6.02 x 1023 N
atoms
a. | 14 | b. | 7 | c. | 0 | d. | none of these |
|
|
|
|
70.
|
What is the mass of 2 moles of He atoms?
a. | 2 g | c. | 8 g | b. | 4 g | d. | 1.20 x 1024
g |
|
|
|
|
71.
|
Which of the following has the greatest mass?
a. | 1 mol of Ag | c. | 1 mol of Au | b. | 1 mol of Al | d. | 1 mol of Ar |
|
|
|
|
72.
|
The percentage composition of carbon in CH4 is about 75%. What ist
he percentage of hydrogen in this compound?
|
|
|
|
73.
|
The approximate mass of one silicon atom is about equal to:
a. | one half the mass of one nitrogen molecule (N2) | b. | the mass of one
nitrogen atom (N) | c. | the mass of one nitrogen molecule
(N2) |
|
|
|
|
74.
|
To calculate the Molarity of a solution, you mus calculate:
a. | liters per grams. | c. | moles per liters. | b. | grams per liters. | d. | iters per
moles. |
|
|
|
|
75.
|
According to the law of conservation of mass, the total mass of the
reactants are
a. | always more than the total mass of the products. | b. | always less than the
total mass of the products. | c. | sometimes more and sometimes less than the
total mass of the products. | d. | always equal to the total mass of the
products. |
|
|
|
|
76.
|
In this equation, which are the reactant(s)?
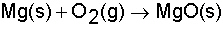 a. |
Mg only | c. | Mg + 02 | b. | Mg0 only | d. | 02 only |
|
|
|
|
77.
|
Which is the name of the kind of solid substance formed in this
figure?
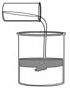 a. | aqueous | c. | coordinate complex | b. | precipitate | d. | synthesis |
|
|
|
|
78.
|
In the reaction represented by the equation N2 + 3H2
® 2NH3, what is the mole ratio of hydrogen to ammonia?
|
|
|
|
79.
|
For the reaction represented by the equation C + 2H2
®
CH4,
how many moles of hydrogen are required to produce 4 mol of methane,
CH4?
a. | 3 mol | b. | 4 mol | c. | 8 mol | d. | 1
mol |
|
|
|
|
80.
|
Which describes the function of a catalyst?
a. | It speeds up a reaction. | c. | It makes a reaction
exothermic. | b. | It slows down a reaction. | d. | It makes a reaction endothermic. |
|
|
|
|
81.
|
The coefficients in a chemical equation represent the
a. | masses, in grams, of all reactants and products. | b. | relative numbers of
moles of reactants and products. | c. | number of atoms in each compound in a
reaction. | d. | number of valence electrons involved in the reaction. |
|
|
|
|
82.
|
What type of chemical reaction releases energy to it’s
surroundings?
a. | endothermic | b. | exothermic | c. | both endothermic and
exothermic | d. | neither endothermic nor exothermic |
|
|
|
|
83.
|
Which type of chemical reaction is shown?
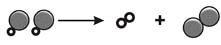 a. | double replacement | c. | synthesis | b. | combustion | d. | decomposition |
|
|
|
|
84.
|
Which type of reaction is Al + O2
® Al2O3?
a. | synthesis | c. | single replacement | b. | decomposition | d. | combustion |
|
|
|
|
85.
|
What are the correct coefficients when this equation is balanced?
____K
+ ____Br2 ®
____KBr
a. | 1, 1, 1 | c. | 2, 1, 2 | b. | 1, 2, 1 | d. | 2, 1, 1 |
|
|
|
|
86.
|
Which type of chemical reaction would this be classified as always producing
carbon dioxide and water like the following:
C3H8
+ O2 ® CO2 +
H2O
a. | synthesis | c. | combustion | b. | double replacement | d. | decomposition |
|
|
|
|
87.
|
How many grams of hydrogen will be required to produce 32.0 grams of methane
from reacting 24 grams of carbon? given:
Hint: plug in the numbers
C +
2H2 ® CH4
|
|
|
|
88.
|
What mass of methane is produced when 36.0 grams of carbon react with 12 grams
of hydrogen?
given:
C + 2H2
® CH4
|
|
|
|
89.
|
When an acid is mixed with a base, __________________ occurs.
a. | a physical change | c. | neutralization | b. | acidification | d. | nothing |
|
|
|
|
90.
|
The following can be indications of an exothermic chemical change during a
reaction where NO HEAT IS ADDED.
a. | bubbles and gas form | c. | both of these | b. | heat is produced | d. | neither of
these |
|