Matching
|
|
|
Match each item with the correct statement below. a. | accuracy | e. | mass | b. | Kelvin temperature scale | f. | absolute zero | c. | Celsius temperature
scale | g. | volume | d. | weight | h. | precision |
|
|
|
1.
|
the closeness to true or accepted value
|
|
|
2.
|
the force of gravity on an object
|
|
|
3.
|
to reproduce a range of measurements that are close together
|
|
|
4.
|
the SI scale for temperature
|
|
|
5.
|
the amount of matter an object contains
|
|
|
6.
|
the amount of space an object occupies.
|
|
|
7.
|
based on the freezing and boiling points of water
|
|
|
8.
|
the lowest point on the Kelvin temperature scale
|
Multiple Choice
Identify the choice that best completes the
statement or answers the question.
|
|
|
9.
|
On the Celsius scale, at what temperature does water freeze?
|
|
|
10.
|
How many meters are there in 1,865 cm?
a. | 186.5 | b. | 18.65 | c. | 0.1865 | d. | 1.865 |
|
|
|
11.
|
The SI unit that is used to measure time is the ____.
a. | kelvin | c. | second | b. | kilogram | d. | meter |
|
|
|
12.
|
Which of the following is an example of a safe laboratory procedure?
a. | eating or drinking from laboratory glassware | b. | tying back long hair
and loose clothing | c. | testing an odor by directly inhaling the
vapor | d. | touching hot objects with your bare hands |
|
|
|
13.
|
0.25 g = ____________ mg
a. | 0.025 | b. | 2500 | c. | 250 | d. | 0.25
|
|
|
|
14.
|
The accepted value is 15.63. Which correctly describes this student’s
experimental data? | Trial | Measurement | | 1 | 12.84 | | 2 | 13.02 | | 3 | 12.96 | | |
a. | precise but not accurate | c. | neither accurate nor
precise | b. | accurate but not precise | d. | both accurate and precise |
|
|
|
15.
|
A volume of 1 milliliter is equivalent to
a. | 1 gram. | b. | 1 cm3 | c. | 10 L3 | d. | 0.5
L |
|
|
|
16.
|
The relationship between the mass m of a material, its volume V,
and its density D is
a. | D = V/m. | c. | D = m/V. | b. | D = mV. | d. | D = m +
v. |
|
|
|
17.
|
A box is 25 cm long, 6 cm wide, and 4 cm high. How many cm3 of water
can it hold?
|
|
|
18.
|
What is the quantity 624.3 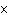 10 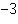 expressed in CORRECT scientific notaion? a. | 62.43 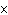 10 10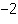 | c. | 6.243 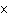 10 10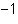 | b. | 6.243 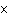 10 10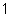 | d. | it is
already in the correct format. |
|
|
|
19.
|
What is the quantity 3.5 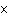 10 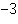 expressed in standard form? a. | 0.0035 | c. | 35000 | b. | 0.035 | d. | 3500 |
|
|
|
20.
|
When designing an experiment, the first step is to ____.
a. | state the problem | c. | list a procedure | b. | state a hypothesis | d. | analyze the
data |
|
|
|
21.
|
The diameter of a carbon atom is 0.000 000 000 154 m. What is this number
expressed in scientific notation?
|
|
|
22.
|
Which of the following observations is qualitative?
a. | A chemical reaction was complete in 2.3 seconds. | b. | It is cold
outside. | c. | The solid had a mass of 23.4 grams. | d. | The pH of a liquid was
5. |
|
|
|
23.
|
1.06 L= ___________ hl
a. | 1060 | c. | 10.6 | b. | 0.0106 | d. | 0.001 06 |
|
|
|
24.
|
A beaker contains 0.32 L of water. What is the volume of this water in
milliliters?
a. | 3.2 mL | b. | 32 mL | c. | 0.32 mL | d. | 320
mL |
|
|
|
25.
|
Which is the correct conversion for a temperature of 67.3°C?
a. | -205.7 K | c. | 19.6° F | b. | 153.1° F | d. | 121.1° F |
|
|
|
26.
|
Objects with a density ________ 1.0 will float in pure water?
a. | less than | c. | equal to | b. | greater than | d. | I have no clue. |
|
|
|
27.
|
Which of the following observations is quantitative?
a. | The liquid is cloudy. | b. | Room temperature is about
25°C. | c. | The liquid tastes bitter. | d. | The liquid turns blue litmus paper
red. |
|
|
|
28.
|
Which is the correct measurement for location marked by the arrow? 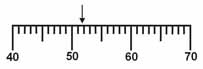
|
|
|
29.
|
The symbols for units of length in order from largest to smallest are
a. | mm, m, cm, km. | c. | km, mm, cm, m. | b. | km, m, cm, mm. | d. | m, cm, mm, km. |
|
|
|
30.
|
The process of using a graduated cylinder to find the volume of an irregular
solid is called the ____ method.
a. | Foster’s | c. | displacement | b. | none of these are correct | d. | scientific |
|