Multiple Choice
Identify the choice that best completes the
statement or answers the question.
|
|
|
1.
|
A substance that is made up of a mixture with particles which are too small to
see is called a:
a. | suspension | c. | heterogenous mixture. | b. | solution. | d. | colloid. |
|
|
|
2.
|
The Law of Conservation of Mass/Matter states that the end products of a
physical or chemical change must have ____________ mass as the starting products.
a. | all of the above. | c. | the same | b. | more | d. | less |
|
|
|
3.
|
A homogeneous mixture can also be called
a. | a compound. | c. | all of the above.. | b. | a solution. | d. | chemically
bonded. |
|
|
|
4.
|
Which of the following is a physical change?
a. | burning a piece of wood | b. | sawing a piece of wood in
half | c. | a copper roof changing color from red to green | d. | rust forming on an
iron fence |
|
|
|
5.
|
If a mixture is uniform in composition (evenly mixed), it is said to be
a. | heterogeneous. | c. | chemically bonded. | b. | a compound. | d. | homogeneous. |
|
|
|
6.
|
A mixture can be classified as a solution, suspension, or colloid based on
the
a. | color of its particles. | c. | number of particles it
contains. | b. | size of its largest particles. | d. | how much liquid it
contains. |
|
|
|
7.
|
If you heat a liquid to find it’s boiling point, you are measuring
a(n)
a. | atomic property. | c. | physical property. | b. | physical change. | d. | molecular
property. |
|
|
|
8.
|
If you describe methane as a gas that easily catches fire, you are describing
a
a. | state of matter. | c. | fire retardant. | b. | chemical property. | d. | chemical
formula. |
|
|
|
9.
|
A substance that is made up of a mixture with particles which are big enough to
see but WILL NOT settle to the bottom is called a:
a. | heterogenous mixture. | c. | solution. | b. | suspension. | d. | colloid.
|
|
|
|
10.
|
A substance that is made up of a mixture with particles so large that they
settle to the bottom (Italian Dressing) is called a:
a. | colloid. | c. | suspension. | b. | solution. | d. | heterogenous mixture. |
|
|
|
11.
|
A compound is
a. | a mixture of elements which can be physically separated. | b. | a substance, made of
two or more atoms that are chemically bonded, that cannot be broken down by physical
means. | c. | any substance, whether it is chemically bonded or not. | d. | the smallest unit of
matter. |
|
|
|
12.
|
Substances that CANNOT be broken down further chemically into other substances
are
a. | mixtures. | b. | compounds. | c. | solutions. | d. | elements. |
|
|
|
13.
|
Which of the following has the highest viscosity?
a. | milk | b. | orange juice | c. | corn syrup | d. | water |
|
|
|
14.
|
The name of the effect which causes light to scatter in fog is called
the:
a. | Tyndall Effect. | c. | magical effect | b. | RV effect. | d. | none of these. |
|
|
|
15.
|
Which of the following is an example of a homogeneous mixture?
a. | air | c. | sour milk | b. | orange juice | d. | vegetable soup |
|
|
|
16.
|
Table sugar(C12H22O11) is an example of
a(n)
a. | atom. | b. | compound. | c. | mixture. | d. | element. |
|
|
|
17.
|
Which of the following is most likely a clue that a physical change has
occurred?
a. | change in shape | b. | smoking and bubbling | c. | formation of a solid
from 2 liquids | d. | production of a gas |
|
|
|
18.
|
One example of a chemical change is
a. | filtering. | b. | boiling water. | c. | crushing a can. | d. | burning
wood. |
|
|
|
19.
|
A physical change occurs when a
a. | reaction forms new substances. | c. | both a and b | b. | no new substances
are formed. | d. | neither a or
b |
|
|
|
20.
|
Which of the following is not a chemical change?
a. | melting | b. | igniting | c. | burning | d. | rusting |
|
|
|
21.
|
Anything that has mass and takes up space is called
a. | homogeneous. | b. | energy. | c. | heterogeneous. | d. | matter. |
|
|
|
22.
|
Soil, a salad, and sugar water are all examples of
a. | elements. | b. | mixtures. | c. | atoms. | d. | compounds. |
|
|
|
23.
|
One example of a physical change is:
a. | burning toast. | c. | dissolving salt in water. | b. | burning
paper. | d. | cooking
eggs. |
|
|
|
24.
|
Matter includes all of the following except
a. | light. | b. | glass. | c. | smoke. | d. | water. |
|
|
|
25.
|
A mixture is
a. | a blend of any two or more types of matter, as long as each part maintains its own
properties. | b. | any group of elements that are chemically bonded to one another. | c. | any substance with a
uniform composition. | d. | a combination of pure substances bonded
chemically. |
|
|
|
26.
|
Which sample shown below shows what would most closely match an
element? 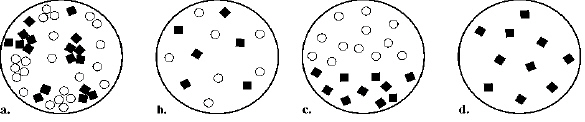
|
|
|
27.
|
Which of the following is an example of a heterogeneous mixture?
a. | sugar | b. | a gold ring | c. | salsa | d. | salt
water |
|
|
|
28.
|
Lemonade, salt water, and Kool=Aid consist of several substances that are NOT
chemically combined, so they are classified as
a. | mixtures. | b. | atoms. | c. | elements. | d. | compounds. |
|
|
|
29.
|
Water is a compound because it
a. | because it is made from 2 types of atoms that are combined
chemically. | b. | can be broken down into hydrogen and oxygen by a chemical change. | c. | neither a or
b. | d. | both a and b |
|
|
|
30.
|
Which of the following is a clue of a chemical change?
a. | iron changes color when heated | b. | bubbles forming and a test tube getting hot
when two things are mixed. | c. | balls of wax form when melted wax is poured
into ice water | d. | gas bubbles form in boiling water |
|