Matching
|
|
|
Match each item with the correct statement below. a. | diatomic | d. | cation | b. | binary compound | e. | anion | c. | polyatomic
ion |
|
|
|
1.
|
a positively charged ion, metals form these.
|
|
|
2.
|
a negatively charged ion, nonmetals form these
|
|
|
3.
|
a molecule made of two identical atoms like H2
|
|
|
4.
|
compound composed of two different elements.
|
|
|
5.
|
group of atoms that are bonded together and carry a + or - charge.
|
|
|
Match each item with the correct statement below. a. | covalent | e. | valence electrons | b. | octet rule | f. | coordination number | c. | ionic
bond | g. | metallic
bond | d. | electron dot diagram |
|
|
|
6.
|
are found in the outermost energy level of an atom
|
|
|
7.
|
a depiction of valence electrons around the symbol of an element
|
|
|
8.
|
Atoms will gain lose or share electrons in order to be like the closest a noble
gas.
|
|
|
9.
|
the force of attraction binding oppositely charged ions together
|
|
|
10.
|
The type of bond formed by two nonmetals.
|
Multiple Choice
Identify the choice that best completes the
statement or answers the question.
|
|
|
11.
|
Which of the following formulas represents a compound whose molecules contain
six shared electrons?
|
|
|
12.
|
Which of the following is NOT a characteristic property of ionic
compounds?
a. | They conduct electricity when dissolved in water. | b. | They form
crystals. | c. | They have high melting points. | d. | They have low melting
points. |
|
|
|
13.
|
Ionic compounds are held together by a force best compared
to________________?
a. | electronic repulsion | c. | both a and b | b. | magnetic attraction | d. | neither a or b |
|
|
|
14.
|
The rule that sates that elements will gain, lose or share electrons in order to
have a full outer shell is called?
a. | The Rule of 2 | b. | Foster’s rule | c. | None of these | d. | Octet
rule |
|
|
|
15.
|
In an electron dot diagram, the dots are used to represent
a. | protons | c. | neutrons | b. | all electrons. | d. | valence
electrons. |
|
|
|
16.
|
What is the chemical name for the compound with the formula
Na2S?
a. | lithium oxide | c. | sodium fluoride | b. | sodium sulfide | d. | magnesium
sulfide |
|
|
|
17.
|
A chemical bond that occurs when atoms share electrons is a(n) ____ bond.
a. | polyatomic | b. | covalent | c. | magnetic | d. | ionic |
|
|
|
18.
|
A single covalent bond contains _____ electrons?
|
|
|
19.
|
A double covalent bond contains _____ electrons?
|
|
|
|
|
|
20.
|
How many valence electrons do MOST Noble Gases have (column H)?
|
|
|
21.
|
How many single covalent bonds can halogens form (Column G)?
|
|
|
22.
|
The chemical formula for water, a covalent compound, is H2O. This
formula is an example of a(n)
a. | molecular formula. | c. | Lewis structure. | b. | ionic formula. | d. | formula unit. |
|
|
|
23.
|
How many covalent bonds can oxygen form?
|
|
|
24.
|
What will happen to Magnesium , Mg when it bonds?
a. | gain 2 electrons | c. | gain 2 protons | b. | gain 6 electrons | d. | lose 2
electrons |
|
|
|
25.
|
What molecular shape does the compound CCl4 have?
a. | octahedral | b. | trigonal planar | c. | bent | d. | tetrahedral |
|
|
|
26.
|
Which element can never have atoms with eight valence electrons even when it
forms bonds?
a. | carbon | b. | nitrogen | c. | oxygen | d. | hydrogen |
|
|
|
27.
|
In what form can an ionic compound conduct electricity?
a. | when warmed slightly | c. | as a crystal | b. | as a solid | d. | when dissolved in
water |
|
|
|
28.
|
How many electrons are needed in the outer energy level of Hydrogen, H for the
atom to be chemically stable?
|
|
|
29.
|
The kinds and the exact number of atoms of each element in a unit of the
compound can be shown in a ____.
a. | chemical symbol | c. | chemical formula | b. | superscript | d. | subscript |
|
|
|
30.
|
What will happen to Oxygen, O when it bonds?
a. | lose 6 electrons | c. | lose 2 protons | b. | lose 2 electrons | d. | gain 2
electrons |
|
|
|
31.
|
How many chloride ions (Cl1-) are needed to balance out the magnesium
ion (Mg1+) create the compound magnesium chloride (MgCl2)?
|
|
|
32.
|
What is the formula for dinitrogen trioxide?
|
|
|
33.
|
In the compound MgCl2, the subscript 2 indicates that
a. | the chloride ion is twice the size of the magnesium ion. | b. | magnesium and
chlorine form a double covalent bond. | c. | there are two chloride ions for each magnesium
ion. | d. | there are two magnesium ions for each ion of chlorine |
|
|
|
34.
|
In the name carbon dioxide, the prefix of the second word indicates that
a molecule of carbon dioxide contains
a. | two oxygen atoms. | c. | a polyatomic ion. | b. | two carbon atoms. | d. | an ionic bond. |
|
|
|
35.
|
What is the correct Lewis structure for hydrogen chloride, HCl? 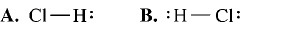 
|
|
|
36.
|
You see a structural formula in which the symbols for elements are connected by
a long dash. You can assume that the chemical bonds in the compound are
a. | covalent. | b. | ionic. | c. | unstable. | d. | metallic. |
|
|
|
37.
|
The sum of the charges in a neutral compound is always ____.
a. | a positive number | c. | a negative number | b. | zero | d. | one |
|
|
|
38.
|
If two covalently bonded atoms are identical, the bond is
a. | polar covalent. | c. | coordinate covalent. | b. | nonpolar covalent. | d. | dipole
covalent. |
|
|
|
39.
|
Why do the noble gases NOT form compounds readily?
a. | They have no electrons. | b. | Their outer energy levels are completely filled
with electrons. | c. | They have seven electrons in the outer energy levels. | d. | They have empty
outer energy levels. |
|
|
|
40.
|
How many single covalent bonds can carbon form?
|
|
|
41.
|
Which is the correct name for the compound Na3P?
a. | Sodium Phosphide | c. | Sodium Triphosphide | b. | Sodium Phosphite | d. | Sodium
Phosphate |
|
|
|
42.
|
What is the correct formula for the compound formed by Mg+2 and
O-2?
|
|
|
43.
|
Study the electron dot diagrams below for lithium, carbon, fluorine, and neon
and choose the correct statement below. a. | Neon will bond with eight lithium’s to form an ionic bond. | b. | Carbon will bond
with four lithium’s to form a covalent bond. | c. | Lithium will lose it’s only valence
electron to fluorine to form a covalent bond. | d. | Lithium will lose it’s only valence
electron and fluorine will accept it to form an ionic bond. |
|
|
|
44.
|
A chemical bond that occurs when atoms share electrons EQUALLY is a(n) ____
bond.
a. | nonpolar | b. | polar | c. | polyatomic | d. | ionic |
|
|
|
45.
|
Which of the following compounds is covalent compound?
|
|
|
46.
|
What kind of chemical bond is formed when a transfer of electrons occurs?
a. | covalent | b. | magnetic | c. | ionic | d. | hydrate |
|
|
|
47.
|
Bonds that form between two nonmetals are usually
a. | covalent. | b. | ionic. | c. | impossible. | d. | weak. |
|
|
|
48.
|
In the 4 molecules: N2, O2, HCl, and F2, which
compound would be polar?
|
|
|
49.
|
The formation of an ionic bond involves the
a. | transfer of positrons. | c. | transfer of electrons. | b. | transfer of
neutrons. | d. | transfer of
protons. |
|
|
|
|
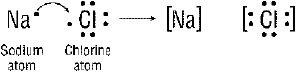
Figure 20-1
|
|
|
50.
|
What is the name of the Figure above?
a. monosodium
monochloride
c. sodium chloride
b. sodium
monchlorine
d. sodium
monochloride
|
|
|
51.
|
What charges are on the sodium (Na) and the chlorine ion (Cl) after the reaction
in Figure 20-1(in the order that they are shown)?
a. 1+ and
1- b. 0 and
1- c. 1- and 1+
d. 0 and 0
|
|
|
52.
|
What happened during the reaction in Figure 20-1?
a. Sodium
gained an electron
c. Chlorine lost 7
electrons
b. Sodium lost an
electron
d. nothing happened
|
|
|
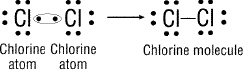
Figure 20-2
|
|
|
53.
|
How is the bond formed in Figure 20-2 above?
a. both chlorines share
electrons. c. both
chlorines lose their electrons.
b. one chlorine takes all of the
electrons. d. there is no bond formed.
|
|
|
54.
|
Do these on the back of your scantron! (7 points)
Using electron dot
diagrams show how bonds are formed for: a. Use arrows to show a
transfer of electrons then draw the resulting ions to show the dot formation for:
MgF2.
Be sure to include the electron dots, the ion
charges and which noble gas EACH is trying to be
like.
b. Use circles
to show sharing and then redraw the structure with dashes to show the formation for:
CH4
H
H
C H
H
THE ATOMS ARE ARRANGED FOR YOU ALREADY.
|