Modified True/False
Indicate whether the statement is true or
false. If false, change the identified word or phrase to make the statement true.
|
|
|
1.
|
The compound with the formula K2O is called potassium
oxygen.
|
|
|
2.
|
When an atom gains an electron it becomes a positive ion.
|
|
|
3.
|
When electrons are transferred between two atoms, a covalent bond is
formed.
|
|
|
4.
|
The attraction between a positive ion and a negative ion results in a
covalent bond.
|
|
|
5.
|
Orderly crystal shapes, high melting points, and electrical conductivity when
dissolved in water are properties of ionic compounds.
|
|
|
6.
|
Molecules are neutral.
|
|
|
7.
|
Low melting points and lack of electrical conductivity are properties of
molecular compounds.
|
|
|
8.
|
Because a water molecule has a slight positive charge at one end and a slight
negative charge at the other end, it is a nonpolar molecule.
|
Multiple Choice
Identify the choice that best completes the
statement or answers the question.
|
|
|
9.
|
Which of the following compounds is covalent compound?
|
|
|
10.
|
In the three molecules, O2, HCl, and F2, what atom would
have a partial negative charge?
a. | fluorine | b. | chlorine | c. | oxygen | d. | hydrogen |
|
|
|
11.
|
What kind of chemical bond is formed when a transfer of electrons occurs?
a. | ionic | b. | covalent | c. | magnetic | d. | hydrate |
|
|
|
12.
|
What is the correct formula for the compound formed by Mg+2 and
O-2?
|
|
|
13.
|
What is the chemical name for the compound with the formula
Na2S?
a. | sodium sulfide | c. | magnesium sulfide | b. | lithium oxide | d. | sodium fluoride |
|
|
|
14.
|
Fluorine, F, forms a binary ionic compound with lithium, Li. What is the name of
this compound?
a. | lithium fluoride | c. | fluorine lithium | b. | fluorine lithide | d. | lithium
fluorine |
|
|
|
15.
|
A double covalent bond contains _____ electrons?
|
|
|
16.
|
You see a structural formula in which the symbols for elements are connected by
a long dash. You can assume that the chemical bonds in the compound are
a. | covalent. | b. | metallic. | c. | unstable. | d. | ionic. |
|
|
|
17.
|
Cations are ____ ions.
a. | charged. | b. | negative. | c. | neutral. | d. | positive. |
|
|
|
18.
|
How many covalent bonds can oxygen form?
|
|
|
19.
|
Which is a property shared by most molecular compounds?
a. | low melting point | c. | nonpolar bonds | b. | high boiling point | d. | high melting
point |
|
|
|
20.
|
A chemical bond that occurs when atoms share electrons EQUALLY is a(n) ____
bond.
a. | polyatomic | b. | nonpolar | c. | ionic | d. | polar
|
|
|
|
21.
|
In the compound MgCl2, the subscript 2 indicates that
a. | there are two chloride ions for each magnesium ion. | b. | there are two
magnesium ions for each ion of chlorine | c. | the chloride ion is twice the size of the
magnesium ion. | d. | magnesium and chlorine form a double covalent bond. |
|
|
|
22.
|
Why do the noble gases NOT form compounds readily?
a. | They have seven electrons in the outer energy levels. | b. | They have no
electrons. | c. | Their outer energy levels are completely filled with electrons. | d. | They have empty
outer energy levels. |
|
|
|
23.
|
What is the correct Lewis structure for hydrogen chloride, HCl? 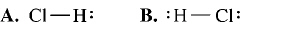 
|
|
|
24.
|
A chemical bond that occurs when atoms share electrons UNEQUALLY is a(n) ____
bond.
a. | polyatomic | c. | nonpolar covalent | b. | polar covalent | d. | polar ionic |
|
|
|
25.
|
When two atoms of the same nonmetal react (like two oxygens or two hydrogens),
they often form a(an)
a. | ionic bond. | c. | diatomic molecule. | b. | polar molecule. | d. | polyatomic ion. |
|
|
|
26.
|
What will happen to Magnesium , Mg when it bonds?
a. | gain 6 electrons | c. | gain 2 protons | b. | lose 2 electrons | d. | gain 2
electrons |
|
|
|
27.
|
Bonds that form between two nonmetals are usually
a. | covalent. | b. | weak. | c. | impossible. | d. | ionic. |
|
|
|
28.
|
The formation of an ionic bond involves the
a. | sharing of electrons. | c. | transfer of electrons. | b. | transfer of
protons. | d. | transfer of
neutrons. |
|
|
|
29.
|
Which element can never have atoms with eight valence electrons even when it
forms bonds?
a. | carbon | b. | nitrogen | c. | oxygen | d. | hydrogen |
|
|
|
30.
|
The kinds and the exact number of atoms of each element in a unit of the
compound can be shown in a ____.
a. | superscript | c. | chemical formula | b. | chemical symbol | d. | subscript |
|
|
|
31.
|
What will happen to Oxygen, O when it bonds?
a. | gain 2 electrons | c. | lose 2 protons | b. | lose 2 electrons | d. | lose 6
electrons |
|
|
|
32.
|
The sum of the charges in a neutral compound is always ____.
a. | a negative number | c. | one | b. | a positive number | d. | zero |
|
|
|
33.
|
Which of the following formulas represents a compound whose molecules contain
six shared electrons?
|
|
|
34.
|
Study the electron dot diagrams below for lithium, carbon, fluorine, and neon
and choose the correct statement below. a. | Neon will bond with eight lithium’s to form an ionic bond. | b. | Carbon will bond
with four lithium’s to form a covalent bond. | c. | Lithium will lose it’s only valence
electron and fluorine will accept it to form an ionic bond. | d. | Lithium will lose
it’s only valence electron to fluorine to form a covalent bond. |
|
|
|
35.
|
A chemical bond that occurs when atoms share electrons is a(n) ____ bond.
a. | magnetic | c. | covalent | b. | ionic | d. | polyatomic |
|
|
|
36.
|
In an electron dot diagram, the dots are used to represent
a. | valence electrons. | c. | neutrons | b. | all electrons. | d. | protons |
|
|
|
37.
|
Which of the following is NOT a characteristic property of ionic
compounds?
a. | They have high melting points. | b. | They have low melting
points. | c. | They form crystals. | d. | They conduct electricity when dissolved in
water. |
|
|
|
38.
|
In what form can an ionic compound conduct electricity?
a. | when dissolved in water | c. | as a crystal | b. | as a
solid | d. | when warmed
slightly |
|
|
|
39.
|
How many electrons are needed in the outer energy levels of most atoms for the
atom to be chemically stable?
|
|
|
40.
|
Ionic compounds are held together by a force best compared
to________________?
a. | electronic repulsion | c. | both a and b | b. | magnetic attraction | d. | neither a or b |
|
|
|
41.
|
In the name carbon dioxide, the prefix of the second word indicates that
a molecule of carbon dioxide contains
a. | two oxygen atoms. | c. | a polyatomic ion. | b. | an ionic bond. | d. | two carbon
atoms. |
|
|
|
42.
|
Anions are ____ ions.
a. | negative. | b. | positive. | c. | neutral. | d. | charged. |
|
|
|
43.
|
A single covalent bond contains _____ electrons?
|
|
|
44.
|
A double covalent bond contains _____ electrons?
|
|
|
45.
|
The rule that sates that elements will gain, lose or share electrons in order to
have a full outer shell is called?
a. | Foster’s rule | c. | Octet rule | b. | The Rule of 2 | d. | None of these |
|
|
|
46.
|
How many chloride ions are needed to cancel the 2+ charge of magnesium in
magnesium chloride (MgCl2)?
|
|
|
47.
|
Which of the following compounds is NOT a covalent compound?
|
For the following questions, type the letter of
the correct answer into the box.
|
|
|
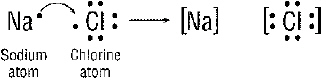
Figure 20-1
|
|
|
48.
|
What charges are on the sodium (Na) and the chlorine ion (Cl) after the reaction
in Figure 20-1(in the order that they are shown)?
a. 1+ and
1- b. 0 and
1- c. 1- and 1+
d. 0 and 0
|
|
|
49.
|
What happened during the reaction in Figure 20-1?
a. Sodium
gained an electron
c. Chlorine lost 7 electrons
b. Sodium lost an electron
d. nothing happened
|
|
|
50.
|
What type of bond is formed in the Figure above?
a.
covalent b.
metallic c.
ionic d. magical
|
|
|
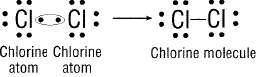
Figure 20-2
|
|
|
51.
|
How is the bond formed in Figure 20-2 above?
a. one chlorine takes all
of the electrons. c. both chlorines lose their
electrons.
b. both chlorines share electrons.
d. there is no bond formed.
|
|
|
52.
|
What type of bond is formed in Figure 20-2 above?
a.
magical b.
metallic c.
ionic d. covalent
|