Matching
|
|
|
Match each item with the correct statement below. a. | absolute zero | e. | mass | b. | Kelvin temperature scale | f. | significant figure | c. | Celsius temperature
scale | g. | precision | d. | weight | h. | accuracy |
|
|
|
1.
|
closeness to true value
|
|
|
2.
|
narrowness of range of measurements
|
|
|
3.
|
the quantity of matter an object contains
|
|
|
4.
|
the lowest point on the Kelvin scale
|
|
|
5.
|
the SI scale for temperature
|
|
|
6.
|
the force of gravity on an object
|
|
|
7.
|
the non-SI scale for temperature
|
Multiple Choice
Identify the choice that best completes the
statement or answers the question.
|
|
|
8.
|
What is the result of adding 2.5 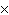 10 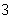 and 3.5 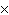 10  ?
|
|
|
9.
|
Which group of measurements is the most precise? (Each group of measurements is
for a different object.)
a. | 2 g, 3 g, 4 g | c. | 1.2 g, 1.4 g, 1.3 g | b. | 2.0 g, 3.0 g, 4.0 g | d. | 1 g, 3 g, 5 g |
|
|
|
10.
|
In the measurement 0.503 L, which digit is the estimated digit?
a. | 5 | b. | 3 | c. | the 0 immediately to
the left of the 3 | d. | the 0 to the left of the decimal
point |
|
|
|
11.
|
Which of the following volumes is the smallest?
a. | one decaliter | b. | one liter | c. | one milliliter | d. | one
deciliter |
|
|
|
12.
|
What is the volume of a salt crystal measuring 2.44 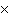 10 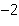 m by 1.4 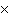 10 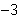 m by 8.4 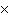
10 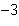 m?
|
|
|
13.
|
What is the temperature of absolute zero measured in 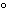 C? a. | –373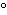 C C | b. | –273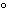 C C | c. | –173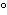 C C | d. | –73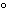 C C |
|
|
|
14.
|
Which temperature scale has no negative temperatures?
a. | Celsius | b. | Fahrenheit | c. | Joule | d. | Kelvin |
|
|
|
15.
|
Which of the following mass units is the largest?
a. | 1 cg | b. | 1 dg | c. | 1 mg | d. | 1 dag |
|
|
|
16.
|
The weight of an object ____.
a. | is the same as its mass | c. | is not affected by
gravity | b. | depends on its location | d. | is always the same |
|
|
|
17.
|
What is the temperature –34 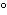 C expressed in kelvins? a. | 139 K | b. | 207 K | c. | 239 K | d. | 339
K |
|
|
|
18.
|
If the temperature changes by 100 K, by how much does it change in  C?
|
|
|
19.
|
A train travels at a speed of 30 miles per hour. If 1 mile = 1.6 kilometers, how
fast is the train traveling in kilometers per minute?
a. | 0.4 km/min | c. | 0.8 km/min | b. | 0.6 km/min | d. | 1.0 km/min |
|
|
|
20.
|
Density is found by dividing ____.
a. | mass by volume | c. | mass by area | b. | volume by mass | d. | area by mass |
|
|
|
21.
|
What is the density of an object having a mass of 8.0 g and a volume of 25
cm 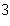 ? a. | 0.32 g/cm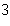 | b. | 2.0 g/cm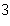 | c. | 3.1 g/cm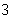 | d. | 200 g/cm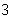 |
|
|
|
22.
|
How is 4.12 x 104 written in standard decimal notation?
a. | 41200 | b. | 0.000412 | c. | 4120 | d. | 0.0412 |
|
|
|
23.
|
Which is the correct measurement for location marked by the arrow? 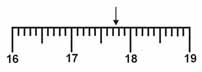 a. | 17.076 | c. | 17.76 | b. | 17.8 | d. | 17.7 |
|
|
|
24.
|
The accepted value is 1.43. Which correctly describes this student’s
experimental data? | Trial | Measurement | | 1 | 1.29 | | 2 | 1.93 | | 3 | 0.88 | | |
a. | accurate but not precise | c. | both accurate and
precise | b. | precise but not accurate | d. | neither accurate nor precise |
|
|
|
25.
|
Which term is described as the amount of matter in an object?
a. | density | c. | mass | b. | volume | d. | length |
|
|
|
26.
|
Which term describes the amount of mass contained per unit volume?
a. | mass | c. | length | b. | temperature | d. | density |
|
|
|
27.
|
An object has a mass of 26.94 grams and a volume of 2.568 cubic centimeters.
What material is it likely to be made of? | Substance | iron | gold | silver | copper | | Density
(g/cm3) | 7.874 | 19.32 | 10.49 | 8.92 | | | | | |
a. | Iron | c. | Silver | b. | Gold | d. | Copper |
|
|
|
28.
|
The product of 2 x 104 cm and 4 x 10–12 cm,
expressed in scientific notation is ____.
a. | 8 x 10–7 cm | c. | 8 x 10–8
cm | b. | 6 x 10–8 cm | d. | 8 x 10–48
cm |
|
|
|
29.
|
A theory is a ____.
a. | proposed explanation for an observation | b. | well-tested
explanation for a broad set of observations | c. | summary of the results of many
observations | d. | procedure used to test a proposed explanation |
|
|
|
30.
|
The accepted value for the density of iron is 7.87 g/cm3. A student
records the mass of a 20.00-cm3 block of iron as 153.8 grams. What is the percent error
for the density measurement?
a. | 19.4% | c. | 2.29% | b. | 7.69% | d. | 2.34% |
|
|
|
31.
|
A bottle contains 3.100 mL of a liquid. The total mass of the bottle and the
liquid together is 6.300 g. The mass of the empty bottle is 4.240 g. What is the density of the
liquid?
a. | 0.665 g/mL | c. | 1.505 g/mL | b. | 1.368 g/mL | d. | 2.032 g/mL |
|
|
|
32.
|
Which of the following observations is quantitative?
a. | The liquid turns blue litmus paper red. | b. | The liquid boils at
100°C. | c. | The liquid tastes bitter. | d. | The liquid is
cloudy. |
|
|
|
33.
|
Which of the following observations is qualitative?
a. | A chemical reaction was complete in 2.3 seconds. | b. | The solid had a mass
of 23.4 grams. | c. | The pH of a liquid was 5. | d. | Salt crystals formed as the liquid
evaporated. |
|
|
|
34.
|
A statement that can be tested experimentally is a
a. | variable. | b. | model. | c. | generalization. | d. | hypothesis. |
|
|
|
35.
|
All of the following are examples of units except
a. | mass. | b. | kilometer. | c. | gram. | d. | ounce. |
|
|
|
36.
|
Which of these is not an SI base unit?
a. | kilogram | b. | second | c. | liter | d. | Kelvin |
|
|
|
37.
|
The symbols for units of length in order from largest to smallest are
a. | m, cm, mm, km. | c. | km, mm, cm, m. | b. | mm, m, cm, km. | d. | km, m, cm, mm. |
|
|
|
38.
|
The unit m3 measures
a. | length. | b. | area. | c. | volume. | d. | time. |
|
|
|
39.
|
A volume of 1 milliliter is equivalent to
a. | 1 cubic centimeter. | c. | 1 liter. | b. | 1 gram. | d. | 10–1 cubic
decimeters. |
|
|
|
40.
|
A change in the force of gravity on an object will affect its
a. | mass. | b. | density. | c. | weight. | d. | kinetic
energy. |
|
|
|
41.
|
The relationship between the mass m of a material, its volume V,
and its density D is
a. | D = mV. | b. | D = V/m. | c. | D = m/V. | d. | D = m +
v. |
|
|
|
42.
|
The density of pure diamond is 3.5 g/cm3. What is the volume of a
diamond with a mass of 0.25 g?
a. | 0.071 cm3 | b. | 0.875 cm3 | c. | 3.75 cm3 | d. | 14
cm3 |
|
|
|
43.
|
10 000 milliliters is equivalent to
a. | 1 hectoliter. | c. | 1 centiliter. | b. | 1 dekaliter. | d. | 1 deciliter. |
|
|
|
44.
|
0.25 g is equivalent to
a. | 250 kg. | b. | 250 mg. | c. | 0.025 mg. | d. | 0.025
kg. |
|
|
|
45.
|
The number of grams equal to 0.5 kg is
a. | 0.0005 | b. | 0.005 | c. | 500 | d. | 5000 |
|
|
|
46.
|
How many minutes are in 1 week?
a. | 168 min | b. | 1440 min | c. | 10 080 min | d. | 100 800
min |
|
|
|
47.
|
If 1 inch equals 2.54 cm, how many centimeters equal 1 yard?
a. | 0.0706 cm | b. | 14.2 cm | c. | 30.5 cm | d. | 91.4
cm |
|
|
|
48.
|
A numerical result is said to have good precision if
a. | it agrees closely with an accepted value. | b. | repeated
measurements agree closely. | c. | it has a small number of significant
figures. | d. | it is a large whole number. |
|
|
|
49.
|
How is the measurement 0.000 065 cm written in scientific notation?
a. | 65 x 10–6 cm | c. | 6.5 x 10–6
cm | b. | 6.5 x 10–5 cm | d. | 6.5 x 10–4
cm |
|
|
|
50.
|
The average distance between the Earth and the moon is 386 000 km. Expressed in
scientific notation, this distance is written as
a. | 386 x 103 km. | c. | 3.9 x 105
km. | b. | 39 x 104 km. | d. | 3.86 x 105 km. |
|
|
|
51.
|
When 1.92 x 10–6 kg is divided by 6.8 x 102 mL, the
quotient equals
a. | 2.8 x 10–4 kg/mL. | c. | 2.8 x 10–8
kg/mL. | b. | 2.8 x 10–5 kg/mL. | d. | 2.8 x 10–9
kg/mL. |
|
|
|
52.
|
The result of dividing 107 by 10–3 is
|
|
|
53.
|
What is the result of multiplying 2.5 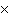 10 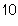 by 3.5 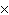 10 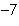 ?
|
|
|
54.
|
How is 2.97 x 10-5 written in standard decimal notation?
a. | 0.000297 | b. | 29700 | c. | 0.0000297 | d. | 297000 |
|
|
|
55.
|
The result of multiplying 105 by 10–2 is
|
True/False
Indicate whether the statement is true or
false.
|
|
|
56.
|
If you wear glasses already, you do not need to wear safety goggles.
|
|
|
57.
|
When moving from a small unit, such as grams, to a larger unit, such as
kilograms, the numerical value will increase.
|
|
|
58.
|
When recording a measurement using an instrument that has markings that
represent the one hundreds place, you must record your answer to the closest ones place.
|
|
|
59.
|
A temperature of 55°F is approximately equivalent to 13°C.
|
|
|
60.
|
A measurement of 5.685 x 10-6 is equivalent to 0.0000005685.
|