True/False
Indicate whether the statement is true(A) or
false(B).
|
|
|
1.
|
When using Charles’s law, temperature must be expressed in degrees
Celsius.
|
|
|
2.
|
Pressure is a direct result of collisions between gas particles and the walls of
their container.
|
|
|
3.
|
According to Charles’s law, the volume of a gas is inversely proportional
to its pressure at constant temperature.
|
|
|
4.
|
A substance does not change in temperature while it is undergoing a change in
state.
|
|
|
5.
|
When the molecules of gases are heated, the average kinetic energy of the
molecules decreases.
|
|
|
6.
|
The combined gas law states the relationship among pressure, temperature, and
volume of a fixed amount of gas.
|
|
|
7.
|
According to Gay-Lussac’s law, pressure is directly proportional to
pressure at constant volume.
|
|
|
8.
|
According to the combined gas law, pressure is inversely proportional to volume
and directly proportional to temperature, and volume is directly proportional to temperature.
|
Multiple Choice
Identify the choice that best completes the
statement or answers the question.
|
|
|
9.
|
As the volume(V) of a gas decreases, the pressure(P) of the gas will ____ if the
temperature remains the same.
a. | remain the same | b. | increase | c. | decrease | d. | contract |
|
|
|
10.
|
According to Gay-Lussac’s law:
a. | volume is directly proportional to temperature at constant
pressure. | b. | pressure is directly proportional to temperature at constant
volume. | c. | pressure is inversely proportional to volume at constant
temperature. | d. | volume is inversely proportional to temperature at constant
pressure. |
|
|
|
11.
|
Matter in which atoms are tightly held in place is a ____.
a. | liquid | b. | solid | c. | plasma | d. | gas |
|
|
|
12.
|
The particles that make up a solid move ____ than do the particles that make up
a gas.
a. | mores slowly | c. | in the same way | b. | more quickly | d. | more quickly and
farther |
|
|
|
13.
|
Which of the following phase changes is an exothermic change?
a. | sublimation | b. | deposition | c. | melting | d. | vaporization |
|
|
|
14.
|
When an inflated balloon is exposed to cold air,
a. | the temperature inside the balloon rises. | c. | the volume of the balloon
decreases. | b. | the pressure inside the balloon rises. | d. | the volume of the balloon
increases. |
|
|
|
15.
|
According to ____, if you decrease the volume of a container of gas and hold the
temperature constant, the pressure of the gas will increase.
a. | Bernoulli’s principle | c. | Archemedes’
principle | b. | Boyle’s law | d. | Charles’s law |
|
|
|
16.
|
According to ____, the volume of a gas increases with increasing temperature, as
long as pressure does not change. (Like a balloon)
a. | Charles’s law | c. | Boyle’s law | b. | Bernoulli’s principle | d. | Archimedes’
principle |
|
|
|
17.
|
During a phase change, the temperature of a substance
a. | increases. | c. | does not change. | b. | increases or decreases. | d. | decreases. |
|
|
|
18.
|
Particles of a liquid
a. | are tightly packed together and stay in a fixed position. | b. | decrease in volume
with increasing temperature. | c. | are free to move within a container but remain
in close contact with one another. | d. | have no
viscosity. |
|
|
|
19.
|
On a long trip, a truck’s tires can get very hot, causing
a. | their pressure to increase. | c. | their pressure to
decrease. | b. | their volume to decrease. | d. | the truck to go faster. |
|
|
|
20.
|
What is the result of a force distributed over an area (f/a)?
a. | temperature | b. | pressure | c. | volume | d. | mass |
|
|
|
21.
|
Matter with no definite volume and no definite shape is a ____.
a. | gas | b. | wood | c. | liquid | d. | solid |
|
|
|
22.
|
A graph that results in a line that indicates a direct relationship between two
variables shows that:
a. | when one variable increases, the other variable remains the same. | b. | both variables are
decreasing. | c. | when one variable increases, the other variable decreases. | d. | when one variable
increases, the other variable increases. |
|
|
|
23.
|
The amount of space that an object takes up is its
a. | density. | b. | mass. | c. | volume. | d. | pressure. |
|
|
|
24.
|
At a temperature of 280 K, the gas in a cylinder has a volume of 20.0 liters. If
the volume of the gas is decreased to 10.0 liters, what must the temperature be for the gas pressure
to remain constant?
a. | 273 K | b. | 560 K | c. | 5600 K | d. | 140
K |
|
|
|
25.
|
An extremely high energy state of matter found in stars and in lightning is
known as _____.
a. | liquid | b. | gas | c. | solid | d. | plasma |
|
|
|
26.
|
Most matter ____ when heated.
a. | expands | b. | condenses | c. | contracts | d. | solidifies |
|
|
|
27.
|
Collisions of helium atoms with the walls of a closed container cause
a. | an overall loss of energy. | c. | gas pressure. | b. | a decrease in
volume. | d. | condensation. |
|
|
|
28.
|
Which of the following phase changes is an endothermic change?
a. | condensation | b. | freezing | c. | deposition | d. | vaporization |
|
|
|
29.
|
As a sample of matter is heated, its particles ____.
a. | stop moving | c. | are unaffected | b. | move more slowly | d. | move more
quickly |
|
|
|
30.
|
Matter that has a definite volume and a definite shape is a ____.
a. | gas | b. | liquid | c. | plasma | d. | solid |
|
|
|
31.
|
Matter that has a definite volume but no definite shape is a
a. | solid. | b. | plasma. | c. | liquid. | d. | gas. |
|
|
|
32.
|
Which state of matter undergoes changes in volume most easily?
a. | gas | b. | liquid | c. | solid | d. | frozen |
|
|
|
33.
|
If the volume of a cylinder is reduced from 8.0 liters to 4.0 liters, the
pressure of the gas in the cylinder will change from 70 kilopascals to
a. | 560 kilopascals. | b. | 35 kilopascals. | c. | 105 kilopascals. | d. | 140
kilopascals. |
|
|
|
34.
|
Which law describes the proportional relationship between the pressure and
volume of a gas?
a. | Natural Law | b. | Boyle’s Law | c. | Charles’s Law | d. | Gay-Lussac’s
Law |
|
|
|
35.
|
As the temperature (T) of a gas increases, the volume(V) of the gas will
____ if the pressure remains the same.
a. | decrease | b. | increase | c. | contract | d. | remain the
same |
|
|
|
36.
|
A sample of neon gas occupies a volume of 752 mL at 25°C. What volume will
the gas occupy at 50°C if the pressure remains constant?
a. | 8150 mL | b. | 815 mL | c. | 408 mL | d. | 204
mL |
|
|
|
37.
|
Amorphous solids:
a. | have no definite melting point | c. | both a and b | b. | include glass and
silly putty | d. | neither a or
b |
|
|
|
38.
|
Raising the temperature of a gas will increase its pressure if the volume of the
gas
a. | and the number of particles are constant. | b. | and the number of
particles are increased. | c. | is increased, but the number of particles is
constant. | d. | is constant, but the number of particles is reduced. |
|
|
|
39.
|
Matter that has a definite volume but no definite shape is a ____.
a. | gas | b. | liquid | c. | plasma | d. | solid |
|
|
|
40.
|
The most abundant stat of matter in the universe
a. | gas | b. | liquid | c. | plasma | d. | solid |
|
|
|
41.
|
According to Boyle’s law, when the pressure of a gas increases at constant
temperature, its volume
a. | increases. | c. | stays constant. | b. | decreases. | d. | increases, then
decreases. |
|
|
|
42.
|
A gas has
a. | no definite shape or definite volume. | b. | a definite volume and definite
shape. | c. | a definite volume but no definite shape. | d. | a definite shape but
no definite volume. |
|
Matching
|
|
|
Match the best answer for each question. Some are not used at all. 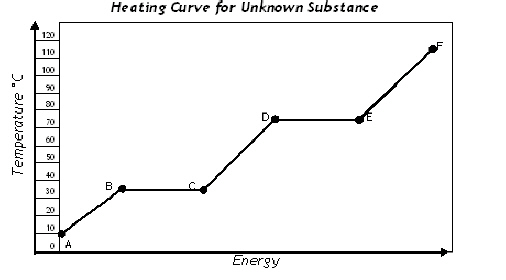 a. | a to b | f. | c to
b | b. | b to c | g. | f to
a | c. | c to d | h. | a to
f | d. | d to e | i. | e to
f | e. | e to d | j. | e to
a |
|
|
|
43.
|
Between which points does condensation occur?
|
|
|
44.
|
Between which points does solid and liquid both exist while gaining
energy?
|
|
|
45.
|
Between which points is this substance a liquid
|
|
|
46.
|
Between which points is this substance a solid
|
|
|
47.
|
Between which points are all of the endothermic processes
|
|
|
48.
|
Between which points are all of the exothermic processes
|
|
|
49.
|
Between which points is this substance a gas
|
|
|
50.
|
Between which points does vaporization occur?
|