True/False
Indicate whether the statement is true or
false.
|
|
|
1.
|
Avogadro’s number refers to the number of particles in one gram of a
substance.
|
|
|
2.
|
One mole of a substance has the same volume as one mole of any other substance,
regardless of what substances are being compared.
|
|
|
3.
|
One mole of a substance has the same number of particles as one mole of any
other substance, regardless of what substances are being compared.
|
|
|
4.
|
To find the percent by mass of a compound if you are given the formula, divide
the molar mass of that element in one mole of the compound by the total molar mass of the
compound.
|
|
|
5.
|
The empirical formula for a compound consists of the symbols for the elements in
the compound without any subscripts.
|
|
|
6.
|
The percent by mass of each element in a compound is known as the percent
composition of a compound.
|
|
|
|
7.
|
The formula for carbon dioxide, CO2, can represent
a. | one molecule of carbon dioxide. | b. | 1 mol of carbon dioxide
molecules. | c. | the combination of 1 atom of carbon and 2 atoms of oxygen. | d. | all of the
above. |
|
|
|
8.
|
What is the formula mass of ethyl alcohol, C2H5OH?
a. | 30.33 amu | b. | 33.27 amu | c. | 45.06 amu | d. | 46.08
amu |
|
|
|
9.
|
The molar mass of NO2 is 46.01 g/mol. How many moles of
NO2 are present in 114.95 g?
a. | 0.4003 mol | b. | 1.000 mol | c. | 2.498 mol | d. | 114.95
mol |
|
|
|
10.
|
The molar mass of CCl4 is 153.81 g/mol. How many grams of
CCl4 are needed to have 5.000 mol?
a. | 5 g | b. | 30.76 g | c. | 769.0 g | d. | 796.05
g |
|
|
|
11.
|
The molar mass of H2O is 18.02 g/mol. How many grams of
H2O are present in 0.20 mol?
a. | 0.2 g | b. | 3.6 g | c. | 35.9 g | d. | 89.9
g |
|
|
|
12.
|
The molar mass of NH3 is 17.03 g/mol. How many moles of
NH3 are present in 107.1 g?
a. | 0.1623 mol | b. | 3.614 mol | c. | 6.289 mol | d. | 107.1
mol |
|
|
|
13.
|
How many oxygen atoms are there in 0.500 mol of CO2?
a. | 6.02 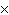 1023 1023 | b. | 3.01 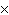 1023
1023 | c. | 15.9994 | d. | 11.0 |
|
|
|
14.
|
How many molecules are there in 5.0 g of methyl alcohol,
CH3OH?
a. | 9.4 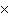 1022 1022 | b. | 3.0 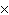 1024
1024 | c. | 3.6 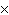 1024 1024 | d. | 3.8 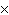 1024
1024 |
|
|
|
15.
|
What is the percentage composition of CF4?
a. | 20% C, 80% F | c. | 16.8% C, 83.2% F | b. | 13.6% C, 86.4% F | d. | 81% C, 19% F |
|
|
|
16.
|
What is the percentage composition of CO?
a. | 50% C, 50% O | c. | 25% C, 75% O | b. | 12% C, 88% O | d. | 43% C, 57% O |
|
|
|
17.
|
The percentage composition of sulfur in SO2 is about 50%. What is the
percentage of oxygen in this compound?
|
|
|
18.
|
A formula that shows the simplest whole-number ratio of the atoms in a compound
is the
a. | molecular formula. | c. | experimental formula. | b. | ideal
formula. | d. | empirical
formula. |
|
|
|
19.
|
What is the empirical formula for a compound that is 31.9% potassium, 28.9%
chlorine, and 39.2% oxygen?
a. | KClO2 | b. | KClO3 | c. | K2Cl2O3 | d. | K2Cl2O5 |
|
|
|
20.
|
What is the empirical formula for a compound that is 43.6% phosphorus and 56.4%
oxygen?
|
|
|
21.
|
A compound contains 259.2 g of F and 40.8 g of C. What is the empirical formula
for this compound?
|
|
|
22.
|
A compound contains 64 g of O and 8 g of H. What is the empirical formula for
this compound?
|
|
|
23.
|
A molecular compound has the empirical formula XY3. Which of the
following is a possible molecular formula?
|
|
|
24.
|
A compound's empirical formula is C2H5. If the
formula mass is 58 amu, what is the molecular formula?
a. | C3H6 | b. | C4H10 | c. | C5H8 | d. | C5H15 |
|
|
|
25.
|
A compound's empirical formula is N2O5. If the
formula mass is 108 amu, what is the molecular formula?
|
|
|
26.
|
A compound's empirical formula is CH. If the formula mass is 26 amu, what
is the molecular formula?
|
|
|
27.
|
What is the mass percentage of chlorine in NaCl?
a. | 35.45% | c. | 60.7% | b. | 50% | d. | 64.5% |
|
|
|
28.
|
To find the molecular formula from the empirical formula, one must determine the
compound's
a. | density. | c. | structural formula. | b. | formula mass. | d. | crystal
lattice. |
|
|
|
29.
|
A compound's empirical formula is CH3. If the formula mass is 30
amu, what is the molecular formula?
|
|
|
30.
|
How many grams of phosphorus are in 500.0 grams of
calcium phosphorus? a. | 49.92 grams | c. | 310.2 grams | b. | 99.84 grams | d. | 130.1 grams |
|
|
|
31.
|
Calculate the number of molecules in 4.0 mol H2O.
a. | 0.60 ´ 1023 molecules | c. | 2.4 ´ 10–23
molecules | b. | 2.4 ´ 1024 molecules | d. | 2.4 ´ 1023
molecules |
|
|
|
32.
|
Which of the following elements exists as a diatomic molecule?
a. | neon | c. | nitrogen | b. | lithium | d. | sulfur |
|
|
|
33.
|
All of the following are equal to Avogadro's number EXCEPT ____.
a. | the number of atoms of bromine in 1 mol Br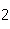 | b. | the number of atoms of gold in 1 mol
Au | c. | the number of molecules of nitrogen in 1 mol N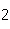 | d. | the number of molecules of carbon monoxide in 1
mol CO |
|
|
|
34.
|
Which of the following compounds has the highest oxygen content, by
weight?
|
|
|
35.
|
What is true about the molar mass of chlorine gas?
a. | The molar mass is 35.5 g. | b. | The molar mass is 71.0 g. | c. | The molar mass is
equal to the mass of one mole of chlorine atoms. | d. | none of the
above |
|
|
|
36.
|
What is the number of moles of beryllium atoms in 36 g of Be?
a. | 0.25 mol | c. | 45.0 mol | b. | 4.0 mol | d. | 320 mol |
|
|
|
37.
|
The volume of one mole of a substance is 22.4 L at STP for all ____.
a. | gases | c. | solids | b. | liquids | d. | compounds |
|
|
|
38.
|
What is the number of moles in 500 L of He gas at STP?
a. | 0.05 mol | c. | 22 mol | b. | 0.2 mol | d. | 90 mol |
|
|
|
39.
|
Given 1.00 mole of each of the following gases at STP, which gas would have the
greatest volume?
a. | He | c. | SO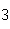 | b. | O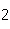 | d. | All would
have the same volume. |
|
|
|
40.
|
The lowest whole-number ratio of the elements in a compound is called the
____.
a. | empirical formula | c. | binary formula | b. | molecular formula | d. | representative
formula |
|
|
|
41.
|
What is the percent composition of carbon, in heptane, C 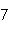 H 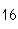 ?
|
|
|
42.
|
Which of the following is NOT an empirical formula?
|
|
|
43.
|
What is the empirical formula of a compound that is 40% sulfur and 60% oxygen by
weight?
|
|
|
44.
|
How many moles of tungsten atoms are in 4.8 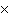 10 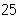 atoms of tungsten?
|
|
|
45.
|
Which of the following is NOT true about empirical and molecular
formulas?
a. | The molecular formula of a compound can be the same as its empirical
formula. | b. | The molecular formula of a compound can be some whole-number multiple of its
empirical formula. | c. | Several compounds can have the same empirical
formula, but have different molecular formulas. | d. | The empirical formula of a compound can be
triple its molecular formula. |
|