Multiple Choice
Identify the choice that best completes the
statement or answers the question.
|
|
|
1.
|
Which precaution should you take when you see this symbol?  a. | do not look directly at the experiment | b. | wear goggles if you are doing the
experiment | c. | wear goggles if you are performing or observing the experiment | d. | watch the teacher
carefully and follow what he or she does |
|
|
|
2.
|
Which of the following is qualitative data?
a. | Color | c. | Temperature | b. | Pressure | d. | Volume |
|
|
|
3.
|
Which of the following is quantitative data?
a. | Color | c. | Shape | b. | Odor | d. | Volume |
|
|
|
4.
|
A theory is a ____.
a. | proposed explanation for an observation | b. | well-tested
explanation for a broad set of observations | c. | summary of the results of many
observations | d. | procedure used to test a proposed explanation |
|
|
|
5.
|
Which of the following is an example of a safe laboratory procedure?
a. | tying back long hair and loose clothing | b. | eating or drinking
from laboratory glassware | c. | touching hot objects with your bare
hands | d. | testing an odor by directly inhaling the vapor |
|
|
|
6.
|
How is 0.00069 written in scientific notation?
a. | 69 ´ 10–5 | c. | 0.69 ´ 10–3 | b. | 6.9 ´
104 | d. | 6.9 ´ 10–4 |
|
|
|
7.
|
Which of the following conversion factors would you use to change 18 kilometers
to meters?
a. | 1000 m/1 km | c. | 100 m/1 km | b. | 1 km/1000 m | d. | 1 km/100 m |
|
|
|
8.
|
What are 6 centimeters equal to?
a. | 600 meters | c. | 60 millimeters | b. | 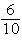 of a
millimeter of a
millimeter | d. | 600
millimeters |
|
|
|
9.
|
Which is the correct measurement for location marked by the arrow? 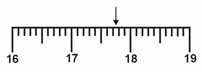 a. | 17.076 | c. | 17.76 | b. | 17.8 | d. | 17.7 |
|
|
|
10.
|
The accepted value is 1.43. Which correctly describes this student’s
experimental data? | Trial | Measurement | | 1 | 1.29 | | 2 | 1.93 | | 3 | 0.88 | | |
a. | accurate but not precise | c. | both accurate and
precise | b. | precise but not accurate | d. | neither accurate nor precise |
|
|
|
11.
|
Which term is described as the amount of matter in an object?
a. | density | c. | mass | b. | volume | d. | length |
|
|
|
12.
|
All of the following are examples of units except
a. | mass. | c. | gram. | b. | kilometer. | d. | ounce. |
|
|
|
13.
|
The symbols for units of length in order from largest to smallest are
a. | m, cm, mm, km. | c. | km, mm, cm, m. | b. | mm, m, cm, km. | d. | km, m, cm, mm. |
|
|
|
14.
|
Which of these metric units is used to measure mass?
|
|
|
15.
|
The quantity of matter per unit volume is
a. | mass. | c. | inertia. | b. | weight. | d. | density. |
|
|
|
16.
|
A change in the force of gravity on an object will affect its
a. | mass. | c. | weight. | b. | density. | d. | kinetic energy. |
|
|
|
17.
|
Which pair of quantities determines the density of a material?
a. | mass and weight | c. | volume and concentration | b. | volume and
weight | d. | volume and
mass |
|
|
|
18.
|
The density of an object is calculated by
a. | multiplying its mass times its volume. | b. | dividing its mass by its
volume. | c. | dividing its volume by its mass. | d. | adding its mass to its
volume. |
|
|
|
19.
|
0.25 g is equivalent to
a. | 250 kg. | c. | 0.025 mg. | b. | 250 mg. | d. | 0.025 kg. |
|
|
|
20.
|
The number of grams equal to 0.5 kg is
a. | 0.0005. | c. | 500. | b. | 0.005. | d. | 5000. |
|
|
|
21.
|
These values were recorded as the mass of products when a chemical reaction was
carried out three separate times: 8.83 g; 8.84 g; 8.82 g. The mass of products from that reaction is
8.60 g. The values are
a. | accurate, but not precise. | b. | precise, but not accurate. | c. | both accurate and
precise. | d. | neither accurate nor precise. |
|
|
|
22.
|
How is the measurement 0.000 065 cm written in scientific notation?
a. | 65 ´ 10–6 cm | c. | 6.5 ´ 10–6 cm | b. | 6.5 ´
10–5 cm | d. | 6.5 ´ 10–4
cm |
|
|
|
23.
|
The measurement 0.020 L is the same as
a. | 2.0 ´ 10–3 L. | c. | 2.0 ´ 10–2 L. | b. | 2.0 ´
102 L. | d. | 2.0 ´ 10–1 L. |
|
|
|
24.
|
How would 0.00930 m be expressed in scientific notation?
a. | 93 ´ 10–4 m | c. | 9.30 ´ 10–3 m | b. | 9.3 ´
10–4 m | d. | 9.30 ´ 10–5
m |
|
|
|
25.
|
When 1.92 ´ 10–6 kg is divided
by 6.8 ´ 102 mL, the quotient equals
a. | 2.8 ´ 10–4 kg/mL. | c. | 2.8 ´ 10–8 kg/mL. | b. | 2.8 ´ 10–5 kg/mL. | d. | 2.8 ´
10–9 kg/mL. |
|
|
|
26.
|
When 6.02 ´ 1023 is multiplied by 9.1
´ 10–31, the product is
a. | 4.3 ´ 10–8. | c. | 4.3 ´ 10–7. | b. | 4.3 ´
1054. | d. | 4.3 ´ 10–53. |
|
|
|
27.
|
The result of dividing 107 by 10–3 is
a. | 10–4. | c. | 104. | b. | 102.5. | d. | 1010. |
|
|
|
28.
|
The capacity of a Florence flask is 250 mL. Its capacity in liters expressed in
scientific notation is
a. | 2.5 ´ 10–2 L. | c. | 2.5 ´ 101 L. | b. | 2.5 ´
10–1 L. | d. | 2.5 ´ 102
L. |
|