Multiple Choice
Identify the choice that best completes the
statement or answers the question.
|
|
|
1.
|
Isotopes are atoms of the same element with different
numbers of _____.
a. | neutrons. | c. | electrons. | b. | protons. | d. | all of these. |
|
|
|
2.
|
Magnesium and calcium are alkaline earth metals, they form
a ____ charge.
|
|
|
3.
|
Which of the following equalities is correct?
a. | 0 K = 273°C | c. | -273 K = 0 °C | b. | 0 K = -273°C | d. | none of these are correct |
|
|
|
4.
|
Which of the following is the correct electron
configuration for carbon?
a. | 1s22p2 | c. | 1s22s12p3 | b. | 1s22s22p6 | d. | 1s22s22p2 |
|
|
|
5.
|
Which of the following uses correct symbols and
coefficients to show the formation of water from gaseous hydrogen and oxygen?
a. | H2(g) +
O(g) ® 2 H2O(l) | c. | 2H2(g) + O2(g) ® 2H2O(l) | b. | 2H2O(l) ® 2H2(g) + O2(g) | d. | 2H2(g) +
O2(g) ® 2 H2O(aq) |
|
|
|
6.
|
When a solid is produced in a chemical reaction that solid is called
a. | a reactant. | c. | a molecule. | b. | a precipitate. | d. | the mass of the
product. |
|
|
|
7.
|
In writing a chemical equation that produces hydrogen gas, the correct
representation of hydrogen gas is
|
|
|
8.
|
What is the whole number that appears in front of a chemical formula in a
chemical equation called?
a. | a coefficient | c. | a subscript | b. | a ratio | d. | a superscript |
|
|
|
9.
|
According to the law of conservation of mass, the total mass of the reacting
substances is
a. | always more than the total mass of the products. | b. | always less than the
total mass of the products. | c. | sometimes more and sometimes less than the
total mass of the products. | d. | always equal to the total mass of the
products. |
|
|
|
10.
|
A chemical equation is balanced when the
a. | products and reactants are the same chemicals. | b. | subscripts of the
reactants equal the subscripts of the products. | c. | same number of each kind of atom appears in the
reactants and in the products. | d. | coefficients of the reactants equal the
coefficients of the products. |
|
|
|
11.
|
In an equation, the symbol for a substance that is dissolved in water is
followed by
a. | (1). | c. | (s). | b. | (aq). | d. | (g). |
|
|
|
12.
|
A chemical formula written over the arrow in a chemical equation
signifies
a. | an impurity. | c. | a catalyst for the reaction. | b. | the formation of a
gas. | d. | a
by-product. |
|
|
|
13.
|
When the equation Fe3O4 + Al ® Al2O3 + Fe is correctly balanced, what is the
coefficient of Fe?
|
|
|
14.
|
Which coefficients correctly balance the formula equation
NH4NO2(s)® N2(g) +
H2O(l)?
a. | 1, 1, 2 | c. | 1, 2, 2 | b. | 2, 2, 2 | d. | 2, 1, 1 |
|
|
|
15.
|
Which coefficients correctly balance the formula equation
C3H7 + O2 ® CO2 +
H2O?
a. | 2, 19, 12, 7 | c. | 4, 19, 12, 14 | b. | 2, 10, 6, 5 | d. | 2, 38, 12, 7 |
|
|
|
16.
|
Which coefficients correctly balance the formula equation CaO + H2O
® Ca(OH)2?
a. | 2, 1, 2 | c. | 1, 1, 1 | b. | 1, 2, 1 | d. | 1, 2, 3 |
|
|
|
17.
|
In what kind of reaction do two or more substances combine to form a new
compound?
a. | double-replacement reaction | c. | decomposition
reaction | b. | synthesis reaction | d. | ionic reaction |
|
|
|
18.
|
The equation CxHy+ O2 ® CO2 + H2O is the general equation for a
a. | synthesis reaction. | c. | single-replacement reaction. | b. | combustion
reaction. | d. | decomposition
reaction. |
|
|
|
19.
|
The equation AX + BY ® AY + BX is the general
equation for a
a. | single-replacement reaction. | c. | decomposition
reaction. | b. | synthesis reaction. | d. | double-replacement reaction. |
|
|
|
20.
|
The equation AX ® A + X is the general
equation for a(n)
a. | double-replacement reaction. | c. | synthesis
reaction. | b. | decomposition reaction. | d. | combustion reaction. |
|
|
|
21.
|
In what kind of reaction does a single compound produce two or more simpler
substances?
a. | single-replacement reaction | c. | decomposition
reaction | b. | ionic reaction | d. | synthesis reaction |
|
|
|
22.
|
The equation A + BX ® AX + B is the general
equation for a
a. | single-replacement reaction. | c. | double-replacement
reaction. | b. | combustion reaction. | d. | decomposition reaction. |
|
|
|
23.
|
The reaction represented by the equation 2Mg(s) + O2(g)
® 2MgO(s) is a
a. | single-replacement reaction. | c. | decomposition
reaction. | b. | double-replacement reaction. | d. | synthesis
reaction. |
|
|
|
24.
|
The reaction represented by the equation Mg(s) + 2HCl(aq) ® H2(g) + MgCl2(aq) is a
a. | single-replacement reaction. | c. | composition
reaction. | b. | decomposition reaction. | d. | double-replacement reaction. |
|
|
|
25.
|
The reaction represented by the equation
Pb(NO3)2(aq) + 2KI(aq) ®
PbI2(s) + 2KNO3(aq) is a
a. | decomposition reaction. | c. | double-replacement
reaction. | b. | synthesis reaction. | d. | combustion reaction. |
|
|
|
26.
|
The reaction represented by the equation 2KClO3(s) ® 2KCl(s) + 3O2(g) is a(n)
a. | decomposition reaction. | c. | synthesis
reaction. | b. | combustion reaction. | d. | ionic reaction. |
|
|
|
27.
|
The coefficients in a chemical equation represent the
a. | number of atoms in each compound in a reaction. | b. | masses, in grams, of
all reactants and products. | c. | number of valence electrons involved in the
reaction. | d. | relative numbers of moles of reactants and products. |
|
|
|
28.
|
A balanced chemical equation allows one to determine the
a. | energy released in the reaction. | b. | mole ratio of any two substances in the
reaction. | c. | electron configuration of all elements in the reaction. | d. | mechanism involved
in the reaction. |
|
|
|
29.
|
The units of molar mass are
a. | amu/g. | c. | g/mol. | b. | mol/g. | d. | amu/mol. |
|
|
|
30.
|
In the reaction represented by the equation N2 + 3H2 ® 2NH3, what is the mole ratio of nitrogen to ammonia?
|
|
|
31.
|
For the reaction represented by the equation C + 2H2 ® CH4, how many moles of hydrogen are required to produce 10 mol
of methane, CH4?
a. | 10 mol | c. | 20 mol | b. | 4 mol | d. | 2 mol |
|
|
|
32.
|
For the reaction represented by the equation 2H2 + O2® 2H2O, how many liters of hydrogen are needed to completely
react 80 grams of oxygen?
a. | 112 liters | c. | 3 liters | b. | 56 liters | d. | 224 liters |
|
|
|
33.
|
For the reaction represented by the equation 2H2 + O2
® 2H2O, how many grams of water are produced from 6.00 mol
of hydrogen?
a. | 6.00 g | c. | 54.0 g | b. | 108 g | d. | 2.00 g |
|
|
|
34.
|
For the reaction represented by the equation 2Na + Cl2 ® 2NaCl, how many grams of chlorine gas are required to react completely
with 2.00 mol of sodium?
a. | 141.8 g | c. | 212.7 g | b. | 70.9 g | d. | 35.5 g |
|
|
|
35.
|
For the reaction represented by the equation 2HNO3 +
Mg(OH)2 ® Mg(NO3)2 +
2H2O, how many grams of magnesium nitrate are produced from 8.00 mol of nitric acid,
HNO3, and an excess of Mg(OH)2?
a. | 445 g | c. | 148 g | b. | 818 g | d. | 593 g |
|
|
|
36.
|
Ozone, O 3, is produced by the reaction represented by the following
equation: 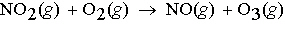 What mass of ozone will form from the reaction of
2.0 g of NO 2 in a car's exhaust and excess oxygen? a. | 1.1 g O3 | c. | 4.2 g O3 | b. | 2.1 g O3 | d. | 1.8 g
O3 |
|
|
|
37.
|
For the reaction represented by the equation Cl2 + 2KBr ® 2KCl + Br2, how many grams of potassium chloride can be
produced from 300. g each of chlorine and potassium bromide?
a. | 98.7 g | c. | 188 g | b. | 451 g | d. | 111 g |
|
|
|
38.
|
For the reaction represented by the equation 2Na + 2H2O ® 2NaOH + H2, how many grams of hydrogen are produced if 120. g
of sodium and 80. g of water are available?
a. | 4.5 g | c. | 45 g | b. | 80. g | d. | 200 g |
|
|
|
39.
|
For the reaction represented by the equation SO3 + H2O
® H2SO4, how many grams of sulfuric acid can be
produced from 200. g of sulfur trioxide and 100. g of water?
a. | 100 g | c. | 200 g | b. | 285 g | d. | 245 g |
|
|
|
40.
|
Which reactant controls the amount of product formed in a chemical
reaction?
a. | excess reactant | c. | limiting reactant | b. | composition reactant | d. | mole ratio |
|
|
|
41.
|
A chemical reaction involving substances A and B stops when B is completely
used. B is the
a. | primary product. | c. | excess reactant. | b. | limiting reactant. | d. | primary
reactant. |
|
|
|
42.
|
For the reaction represented by the equation C + 2H2 ® CH4, how many molecules of hydrogen are required to produce
1.5 mol of methane, CH4?
a. | 1 mole | c. | 9.0 x 10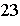 molecules molecules | b. | 1.8 x 10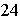 molecules molecules | d. | 3
moles |
|
|
|
43.
|
The symbol D when found in a chemical reaction stands
for
a. | reactants | c. | energy | b. | ice | d. | products |
|
|
|
44.
|
In an exothermic reaction
a. | heat is given off as a product | c. | thermal energy is needed by
reactants | b. | all of these are possible | d. | heat is required to start the reaction |
|
|
|
45.
|
When the symbol D is found to the left of the yields
sign (®), the reaction must be
a. | exothermic | c. | endothermic | b. | in water | d. | slow |
|